9 Oct 2018
Andy Forbes discusses some aspects of helminth infections in these cattle, and explains how adequate levels of control can be achieved with relatively little anthelmintic inputs.
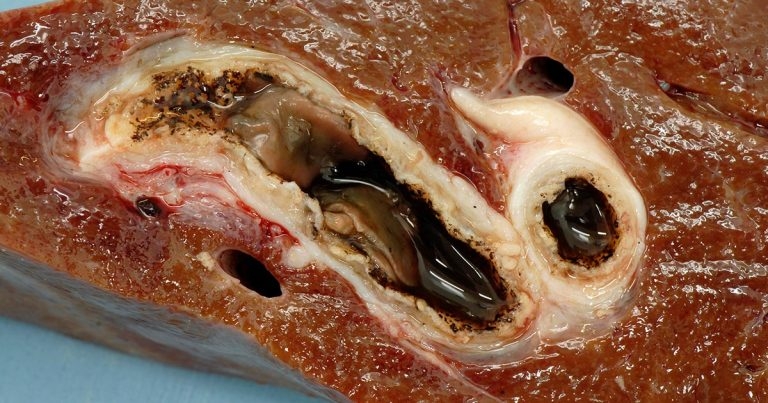
Figure 1. A cross-section of bovine liver showing adult liver fluke (Fasciola hepatica), and fibrosed and calcified bile ducts.
Many classical studies on the epidemiology, impact and control of helminth parasites of cattle have been focused on autumn/winter-born, weaned calves sourced from dairy herds (Urquhart et al, 1987).
Furthermore, research into the adverse effects of helminths in adult cattle has been biased towards dairy cows, rather than beef (Charlier et al, 2009).
While some findings from dairy-oriented research can reasonably be extrapolated to beef cattle, several aspects of parasitology should be specifically addressed by veterinary clinicians when offering advice to beef farmers.
This article focuses on some features of helminth infections in beef cattle; additional information, including control of ectoparasites, can be found in a corresponding paper in Cattle Practice.
Competence in providing advice on parasitology requires a good grasp of the basic information (Forbes, 2012) underpinning the scientific evidence base for control.
It is impossible to provide rational guidance to farmers without knowledge and understanding of subjects such as:
Practical parasitology is essentially the application of this knowledge to individual farms, and farmers, to ensure stock remains healthy and achieves performance targets. At the same time, “big picture” considerations – including parasiticide resistance, environmental impact and carbon footprint – need to be incorporated into plans and proposals for parasite control, but without compromising animal welfare.
Beef breeding herds are, commonly, low input systems that take advantage of the natural reproductive cycles of bovids, which are geared towards calving shortly before the onset of maximum vegetation growth (Wilson and Mittermeier, 2011), which, in turn, provides dams with high levels of nutrition to support lactation and suckling calf growth. Thus, spring calving is the most common husbandry type for beef herds in the British Isles (Gates, 2013).
Spring-born calves have some advantages from a parasitological aspect as well, insofar as:
Additionally, although it is wrong to think of adult cows solely as “sweepers/hoovers” of infective larvae (Stromberg and Averbeck, 1999), their immunity to Cooperia oncophora results in low faecal egg output of this species and, therefore, reduced exposure in their non-immune calves. Conversely, cows can be an important source of pasture contamination with eggs of Ostertagia ostertagi, Dictyocaulus viviparus and Fasciola hepatica.
In North America, beef cows are commonly treated for gastrointestinal nematodes pre-calving; the rationale rests on reducing shedding of helminth eggs on pasture and increasing milk yield. Several studies have shown this practice can increase weaning weight in calves, compared to those from untreated dams (Hawkins, 1993). In the UK, where most (adult) cattle are routinely housed over winter – and frequently treated with anthelmintics for gastrointestinal nematodes, lungworm and liver fluke (F hepatica) at this time – such benefits may also accrue by default.
On liver fluke-endemic farms with high morbidity in the herd, a strategic treatment of the cows with a flukicide around June/July can be included in the control regime (Parr and Gray, 2000). The purpose of this is to remove adult fluke burdens (Figure 1) so minimal pasture contamination with fluke eggs occurs over the period when snail populations typically expand, thus reducing the risk of high cercarial output in the autumn (Over, 1982).
Although somewhat dependent on the interval between any previous treatments – and the spectrum of the flukicide used at the time – treatment with an adulticide is generally sufficient at this time, as this can limit egg output for four to eight weeks (Forbes et al, 2015), which should cover the period of optimal snail activity.
Although the risk of acquiring pasture-borne endoparasite infections in beef suckler calves is typically low, the exception is coccidiosis, which is common in beef calves about two months of age (SAC Consulting, 2018); vigilance and prompt treatment are required in herds where infection is endemic.
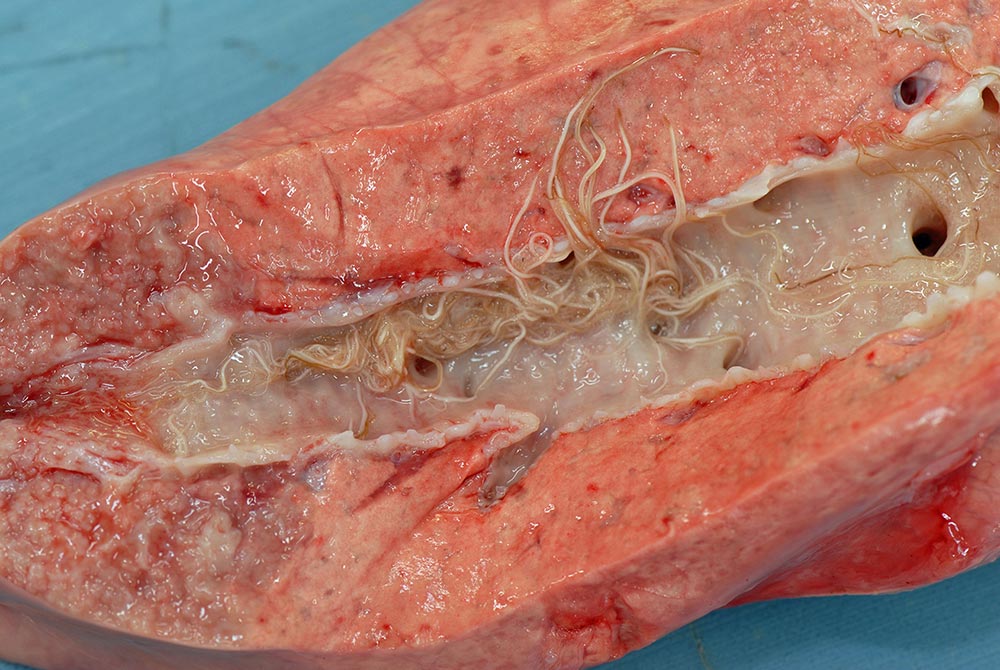
Similar approaches are appropriate for lungworm (Dictyocaulus viviparus; Figure 2), although parasitic bronchitis (PB) is much more common in calves post-weaning. In pre-weaned beef calves running with their dams, while anthelmintics can deliver production responses (Forbes et al, 2002), routine treatment for parasitic gastroenteritis (PGE) is generally unnecessary if daily growth rates exceed 1kg per day.
Post-weaning, calves no longer have a milk-based diet and, consequently, derive all their nutrients from grazed grass, with inevitably higher exposure to infective larvae and possibly fluke metacercariae. The course of action to follow with weaned calves is dependent on several factors, including:
Infective larvae and metacercariae generally survive poorly in silage, and hay and cycling of infections in yards or cubicles is very limited – so housing provides a good opportunity for the control of helminth parasites of cattle.
The administration of anthelmintics at housing can remove existing infections and prevent disease later in the winter. Unless recently treated, all cattle will harbour gastrointestinal parasites – particularly O ostertagi – as animals that have grazed for several months should be immune to Cooperia species.
A risk in young cattle that have acquired high populations of inhibited O ostertagi larvae in the autumn is ostertagiosis type two – an acute, occasionally fatal gastritis resulting from the simultaneous emergence of adult worms in late winter. To prevent ostertagiosis type two, housing treatments with anthelmintics effective against inhibited larvae are recommended.
Scientific evidence indicates MLs have consistently high efficacy and the benzimidazoles have variable efficacy (Williams et al, 1997), but levamisole has no activity against inhibited O ostertagi larvae (Williams, 1991). All cattle anthelmintics have good activity against D viviparus, so a housing treatment should be effective in removing lungworms and minimising the risk of parasitic bronchitis over winter.
Advice on treatment for liver fluke in cattle at housing is frequently empirical, based on:
Surveys in Europe have indicated that adults predominate in liver fluke populations present in older cattle in the autumn (Charlier et al, 2008; MacGillivray et al, 2013). This is logical if animals have been exposed to infection for six months or longer, and have not been treated with a flukicide less than three months prior to housing, because adult liver fluke are long-lived – two years or more in untreated cattle (Ross, 1968) – and juvenile fluke can experience high mortality in the bovine liver (Ross, 1965).
In a comparative efficacy study in weaned, spring-born, beef suckler calves – where, due to their age and grazing history, it was likely they harboured a balanced, mixed-age fluke population – efficacy against F hepatica essentially reflected the profiles of the various products (Forbes et al, 2015). Fluke eggs reappeared in the dung of a small proportion of animals treated with nitroxynil and clorsulon 8 weeks to 12 weeks after treatment; no fluke eggs were detected in any samples from triclabendazole-treated cattle. Growth rates in the cattle were not different among the treatment groups, although, overall, infection with fluke reduced daily live weight gain. Therefore, the choice of flukicide and timing of treatment at/during housing may not be as critical as previously thought.
Although the foundations of long-term health and performance are established in the first year of life (Wathes et al, 2014), it is important that monitoring of young cattle continues into their second grazing season to ensure helminth parasites do not impact on the ability of heifers to reach minimum breeding weight by the age of 15 months, to enable calving at two years of age or beef animals to be marketed and finished efficiently.
On farms with a history of lungworm, yearlings can be vaccinated against D viviparus before turnout. This may be of particular value in replacement heifers to ensure some level of enzootic stability.
Beef yearlings – especially late-born, light cattle – can also benefit from a limited strategic anthelmintic programme to control PGE (Taylor et al, 1995; Figure 3). Depending on history and local circumstances, strategic treatments for F hepatica can also be considered in this age group of cattle.

Although beef cattle enterprises are typically low-input systems, this does not mean parasites can be ignored, even though spring-calving systems generally carry a lower risk for PGE and PB compared with many dairy herds, particularly for youngstock.
Adequate levels of helminth control can be achieved with relatively modest anthelmintic inputs, in which housing treatments are an important component.
Adult breeding animals should not be excluded from strategic actions, particularly in terms of fasciolosis; nor should bulls be forgotten, as they may be more susceptible to parasites than cows (Herd et al, 1992).