19 Nov 2018
Preparing rams for mating part 2 – pre-breeding soundness exams
Jenny Hull, following part one of her article, discusses the benefits and stages of fertility testing male sheep.

Figure 1. Testicles, when palpated, should move freely in the scrotum.
On farms where rams are used together in groups of two or more and pre-breeding soundness exams (PBSEs) are not carried out, a ram can be purchased for a potentially large sum and kept for several breeding seasons without leaving a lamb. This is costly, inefficient production.
Fertility testing of newly purchased rams will safeguard investment, and testing older animals will remove subfertile and infertile rams from the farm. This is imperative for single sire mating groups, which are gaining in popularity as more farms look to breed their own replacements and are using selective breeding to improve their flock.
Published work involving the pre-breeding examination of 280 rams across 5 practices showed 16% of those examined were considered unsuitable for breeding (Lovatt et al, 2016). Major abnormalities noted included those to feet (17.6%), teeth (12%) and the rest of the body (5.5%).
Reproductive abnormalities, meanwhile, included those to the penis (1.9%), scrotum (3.9%) and prepuce (1.1%). A total of 7.6% of testicles were too soft (of poor resilience), while 5.25% were too small.
Fertility testing is a good use of vet time on farm and offers a good opportunity to look over the ram stud, including observing lameness levels and so on. Barr (1984) showed five years of routine PBSEs of all rams on a farm resulted in eliminating a 17% infertility problem and increasing the lamb crop by 2.27%.
Ultimately, fertility testing rams is about improving production efficiency and reducing costs by only keeping and using those that are fertile and fit for purpose. This will be evermore crucial going forwards in a post-subsidies era of British farming. Ideally, rams should be semen tested at least two months pre-tupping (Boundy, 1993). This allows time to correct any body condition and lameness issues, and retest any failures, allowing for the six weeks taken for sperm production. It also allows time to source any replacement rams should they be needed.
It is worth bearing in mind that, in early lambing flocks, rams examined out of season will potentially have a substantial reduction in size and weight of the testicles, and sperm production will also be reduced (Boundy, 1993).
Treatment of rams with melatonin implants can increase scrotal diameters and testicular volumes – and, therefore, fertility of rams out of season (Cevik et al, 2017).
General examination
A general clinical examination of each ram should be carried out and the findings recorded on, for example, the Sheep Veterinary Society (SVS) pre-breeding examination on-farm data collection form (SVS, 2014a).
A reasonably thorough examination can be carried out quite quickly, adopting a nose-to-tail approach, including the following:
- Dentition. Check the incisors – do they meet the dental pad or is the ram undershot or overshot, and are any incisors missing?
- Check the neck area for possible abscesses associated with caseous lymphadenitis.
- Palpate the brisket for sores. Fthenakis et al (2001) showed potentially 7% of rams at a PBSE will have a brisket sore and it is associated with general health abnormalities of rams that affects reproductive performance. Rams with brisket sores will often be concurrently lame.
- Body condition score (BCS) the ram (1 = emaciated, 5 = obese; Agriculture and Horticulture Development Board Beef and Lamb, 2018). Rams of BCS 1 to 2 can be a sign of poor dentition, concurrent diseases such as Johne’s disease, liver fluke or ovine pulmonary adenocarcinoma.
- Legs – observe limb conformation as straight hocks and sloping pasterns will reduce the rams’ working life (Vipond and Greig, 2007; Greig, 2007).
- Feet – rams need to be turned over to properly examine all four feet for signs of interdigital granulomas, toe granulomas, footrot, contagious ovine digital dermatitis or scald. Lovatt et al (2016) showed 17.6% of rams presented for a PBSE had feet abnormalities. Turning the ram is best done after semen collection to reduce stress prior to doing so. Hopefully any problems with lameness and general body condition have been corrected by the time it comes to fertility testing.
Reproductive examination
Examination of the reproductive system with the ram turned over should include the following:
- Testicles should be smooth, firm (resilience), of equal size and, when palpated, move freely in the scrotum (Figure 1). See Table 1 for suggested minimum scrotal circumference measurements (Figure 2).
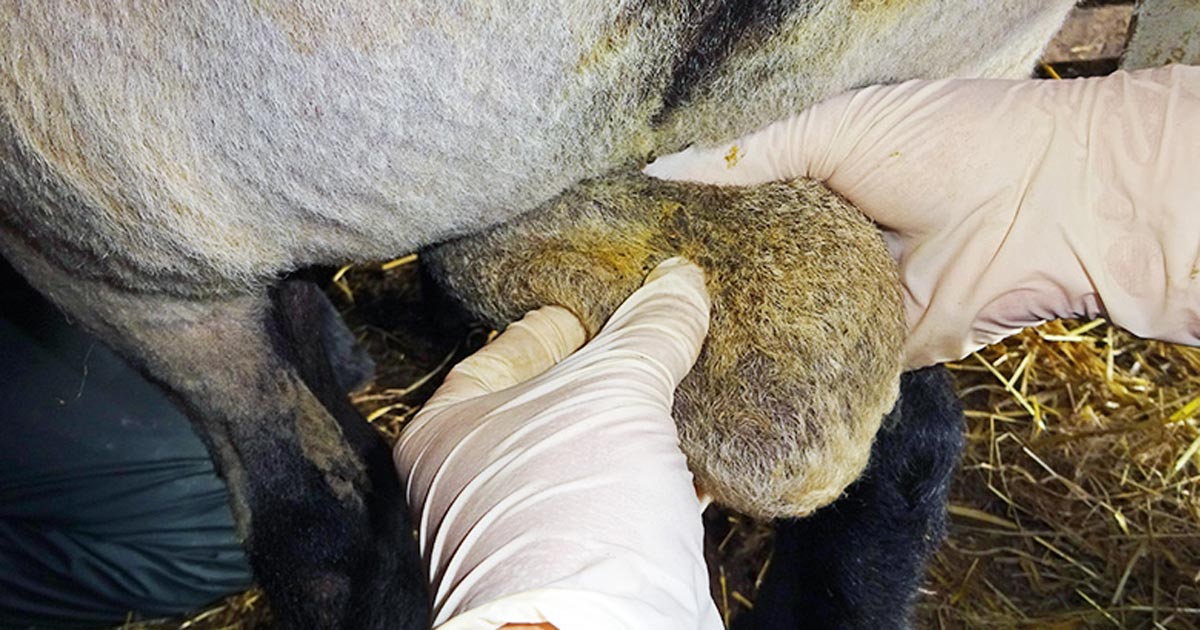
Figure 1. Testicles, when palpated, should move freely in the scrotum. 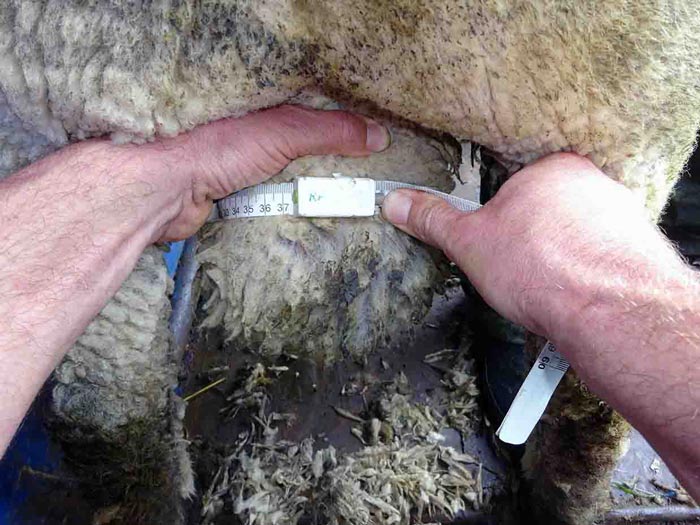
Figure 2. Measuring scrotal circumference. - Epididymis should feel slightly firmer than the testicles, with no lumps, heat, pain, swelling or unevenness (Figure 3). Epididymitis of the head, characterised as a hardened thickening, is quite a common finding.
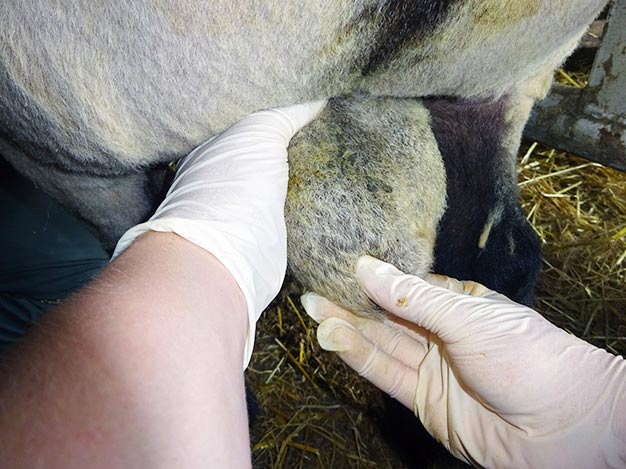
Figure 3. Palpating the tail of the epididymis. - Spermatic cords should be palpated for a mass, such as an abscess or varicocele.
- The scrotum should be clean, with no evidence of chorioptic mange. The effect of a woolly scrotum causing testicular overheating in a UK climate is debatable.
- The prepuce and penis should be examined for any signs of disease or trauma. A very floppy prepuce may reduce mating ability. The penis should be extruded and examined for signs of disease, such as balanitis (Figure 4), or an abnormal or missing urethral process. A missing urethral process in itself may not reduce fertility, but in a newly purchased ram, may be a sign of an episode of urolithiasis previously.
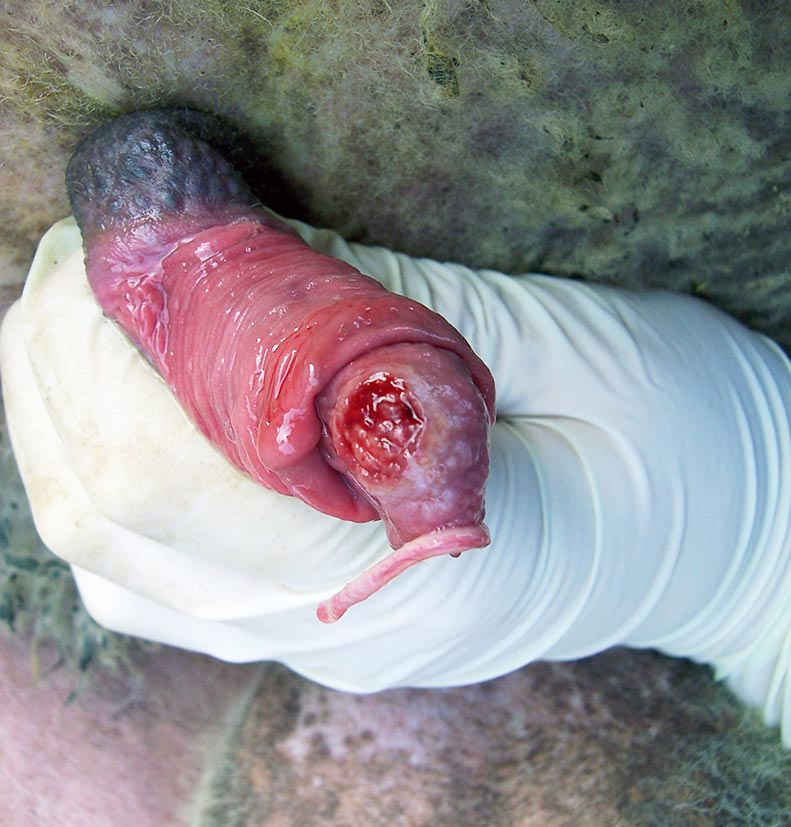
Figure 4. The penis should be extruded and examined for signs of disease such as balanitis.
| Table 1. Minimum scrotal circumference measurements | |||
|---|---|---|---|
| Ram lamb (<1 year old) | Shearling ram (1-2 years old) | Mature ram (>2 years old) | |
| Down or longwool | 30cm | 32cm | 36cm |
| Hill breeds | 28cm | 30cm | 34cm |
| (Sheep Veterinary Society Ram Fertility Workshop, Edinburgh, 2014) | |||
It is not uncommon to find congenital abnormalities in rams at a PBSE, including those that have been sold through a breeding sale. A study of 7,307 rams at slaughter (Smith et al, 2012) showed a prevalence of congenital lesions of 2.21%. Lesions included:
- testicular hypoplasia
- microtestes
- notched (or split) scrotum
- hypospadias
- cryptorchid
- ectopic testis
- scrotal hernia
- epidydimal lesions such as segmental aplasia, cyst, blind efferent duct and sperm granulomas
Many of these abnormalities will affect fertility, but also important is whether these animals with potentially inheritable conditions should be used for breeding at all.
Any abnormalities of the reproductive exam detected should be recorded at the time of examination, along with their significance in terms of the ram’s reproductive potential.
Semen collection and analysis equipment
Three types of electro-ejaculator are commonly available for ram semen collection:
- Lane Pulsator IV Auto Adjust fitted with a ram probe (Figure 5)
- Lane ram ejaculator (Figure 6)
- Ruakura-type ram ejaculator

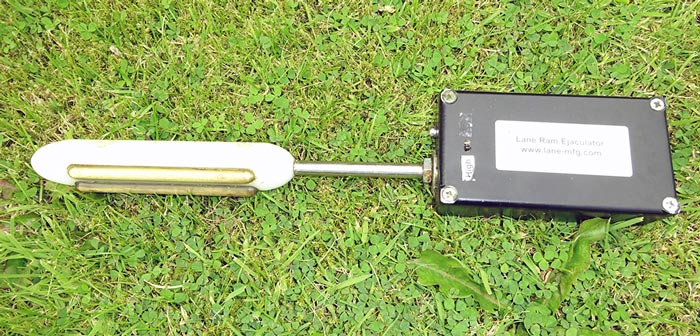
The Ruakura-type probe has annular electrodes that also stimulate spinal nerves, leading to painful back and hindlimb muscle stimulation, and, in the author’s opinion, should not be used on welfare grounds. Its use has long been surpassed by the other types of probe. The Lane Pulsator IV Auto Adjust is commonly used for bull semen testing and can be fitted with a ram probe. The handheld Lane ram ejaculator is the author’s preferred choice, due to it being much quicker and, therefore, the least stressful way to collect a semen sample.
The ram fertility testing box should contain the same as a bull testing kit, except for the addition of a ram probe (Panel 1).
Warm box
- Microscope slides
- Microscope cover clips
- Marker pen
- Pipettes
- Warmed phosphate buffered saline
- Warmed nigrosin-eosin stain
- Slide containers
- Plastic collecting bags such as catering piping bags
- Insulin syringes (for nigrosin-eosin stain)
Cold box
- Ram probe/lanes ram ejaculator
- Collection handle
- Lube
- Paper towel
- Clipboard, pre-breeding soundness exam forms and pen
- Scrotal tape measure
- Microscope
- Heated stage
- Latex gloves
A cost-efficient way of making a warm box for semen analysis is use of a polystyrene box into which four 1L bottles filled with hot water (not boiling) are placed in the bottom. It is important to remember everything that comes into contact with the semen sample must be warm (to prevent cold shock), but not hot (to prevent heat shock); this includes use of a heated stage for the microscope. Heated stages can be retrofitted to any microscope (Figure 7).

Semen collection and analysis
SVS advice (2014b) has recommended routine electro-ejaculation and evaluation of semen provides appropriate additional information when rams are to be used in high-pressure situations, such as single sire mating or with large numbers of ewes, but that it may not be appropriate in low-pressure situations.
In the author’s opinion, a semen sample should be collected from all rams presented for a PBSE, as subfertile and infertile animals can present without any physical abnormalities. A study suggested 9% of rams with no detectable physical abnormalities failed a PBSE due to a poor semen sample (Lovatt et al, 2014).
Semen collection is possible using an artificial vagina, but can be difficult and slow in unhandled and stressed rams. It is often simpler – and much quicker – to perform semen collection by electro-ejaculation, although the sample collected may not always be representative.
Animals should be either haltered and/or manually restrained against a solid fence, or cattle stocks can be used (Figure 8). If using the Lane ram ejaculator, it is best used following the manufacturer’s instructions, which involves massaging the prostate 10 times, then administering 4 seconds of current before massaging the prostate another 10 times and repeating this cycle.

In many cases, a sample is collected after only one or two cycles. If a sample has not been produced after four cycles, the ram should be rested and another attempt to collect a sample made at least 10 minutes later, often at the end of the group. The Lane Pulsator IV can be set to run on an automatic programme as for bulls, but is much quicker used in the same way as the aforementioned Lane ram ejaculator, although it is still slower than using the handheld Lane ram ejaculator.

Once collected (Figure 9), the sample is then immediately analysed for the following:
- Gross density – visual assessment of the density of the sample graded from 0 to 5 (0 = water, 5 = thick cream; Figure 10).

Figure 10. Visual assessment of gross density. - Gross motility – under the microscope, at low power, one drop of semen is placed on a warmed microscope slide (Figure 11) and gross motility is assessed, looking at wave motion – again, graded from 0 to 5 (0 = no activity, 5 = looks like a pint of Guinness settling; Figure 12).

Figure 11. One drop of semen is placed on a warmed microscope slide. 
Figure 12. Gross motility score 5 out of 5. - Progressive motility – one very small drop of semen is then diluted with warmed phosphate buffered saline and covered with a cover slip to assess the percentage of sperm actively going forwards. This should be performed under medium or high power, with the condenser at its lowest setting. Phase contrast is useful, but not essential.
- Morphology – one very small drop of semen is added to two drops of nigrosin-eosin stain on a microscope slide and spread similar to a blood smear (Figure 13). Once dry, morphology is assessed under oil immersion by counting 100 sperm and noting the number of deformities, such as detached or deformed heads, retained midpiece, cytoplasmic droplets and bent or coiled tails.
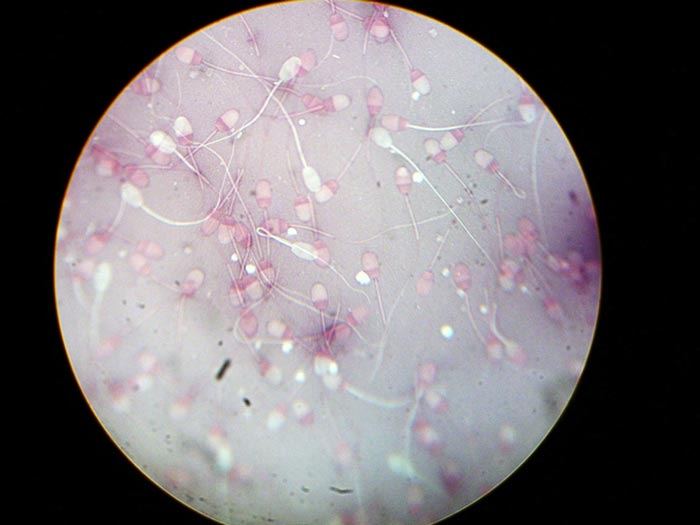
Figure 13. Negrosin-eosin stained semen for morphology assessment.
If a concern of infection exists, the sample can be checked for leukocytes by being air dried, heat fixed and stained, with new methylene blue (similar to an anthrax slide preparation). The presence of large numbers of neutrophils would suggest an inflammatory condition that will affect fertility (Boundy, 1993). It is worth noting if collection of semen with the penis retained in the prepuce will result in greater odds of leukospermia relative to semen collection with the penis extended (Van Metre et al, 2012).
A sample with a minimum score of 3/5 for density and 3/5 for gross motility, with 60% progressive motility and 70% morphologically normal is a pass (SVS, 2014) and therefore deemed fit for breeding. Rams with a low score – for example, density score 2 and motility score 2 – will be subfertile and, therefore, leave some lambs and could potentially be used as part of a team of three if the other two rams are fertile. This PBSE prevents three subfertile rams being used together.
Conventionally, the reproductive examination is done first, followed by semen sampling. In the author’s opinion, this stresses the ram just before sampling and he is subsequently less likely to produce a semen sample. Therefore, it is preferable, in the author’s opinion, to semen test first, followed by a reproductive examination.
Further investigation
Rams that fail to produce a semen sample, or produce a poor sample that has no physical abnormalities found, should be retested a minimum of two weeks later. No ram should be culled on a single, poor-quality semen sample if no other physical problems are detected.
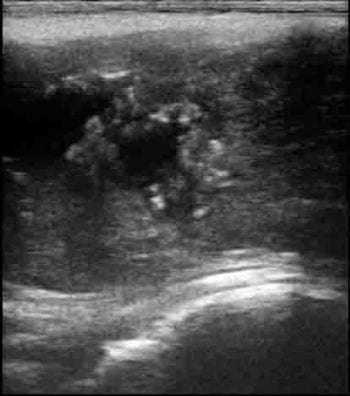
Masses should be further investigated using ultrasound with or without ultrasound-guided needle aspiration for diagnosis of an abscess. Varicoceles, abscesses and spermatoceles can be diagnosed by ultrasound (Figure 14).
Libido
PBSEs are not a diagnosis of libido, and this should be explained to farmers and recorded on the PBSE examination form. The prevalence of same sex-orientated rams has been estimated at 8% (Roselli and Stormshak, 2009).
