9 Sept 2019
Neil Sargison discusses principles of control, as well as challenges in the face of gastrointestinal roundworm and lungworm adaptation.
Dictyocaulus viviparus. Image: Alan R Walker / CC BY-SA 3.0

Gastrointestinal roundworms and lungworms are foremost causes of production loss in cattle – wherever seasonally warm and humid climatic conditions, and agricultural management, provide various opportunities for the survival and development of their free-living stages, and completion of parasitic life cycles.
Their impact – resulting in animal deaths, diarrhoea, respiratory disease, reduced milk yields and illthrift – is greatest under intensive pastoral management systems that are conducive to high levels of infectious larval challenge.
Therefore, control of these parasites is a prerequisite for efficient livestock production, and affords a pragmatic method of engagement with commercial farmers on the broader topic of improved production efficiency through planned animal health management.
The principal gastrointestinal nematode species causing disease and production loss in cattle kept in temperate environments is Ostertagia ostertagi; whereas nematode parasites belonging to Cooperia, Trichostrongylus, Haemonchus and Nematodirus genera may be important in specific scenarios.
O ostertagi is host-specific for bovids and causes pathology by evoking host immune responses, mainly as larval stages invade or leave abomasal glands.
Lungworm is caused by Dictyocaulus viviparus evoking host hypersensitivity responses as parasitic stages migrate through the lungs, before residing in the major airways.
Host protective immunity requires exposure to infective larvae (L3) in particular – and is slow in onset, but long-lasting, in the case of O ostertagi; and rapidly acquired, but requiring regular boosting in the case of D viviparus.
Dairy calves and most autumn-born beef suckler calves are usually weaned from their dams before being turned out to grass for the first time, whereas spring-born beef suckler calves spend their first grazing season alongside their dams.
In the absence of control measures, immunity to O ostertagi is not acquired until the end of dairy or autumn-born beef suckler calves’ first grazing season. In the case of spring-born beef suckler calves, suckling limits the opportunity for L3 exposure during the first half of the first grazing season, while their immune dams effectively remove any L3 contamination from the pasture without developing epidemiologically important patent infections.
The onset of immunity to D viviparus depends on the timing of first challenge, which may typically arise during early summer in warmer regions in the Republic of Ireland, or during the autumn in colder parts of Scotland.
Gastrointestinal roundworms and lungworms have direct life cycles (Figure 1), in which adults are dioecious and sexually reproducing, shedding eggs that hatch and develop through larval stages in the environment.
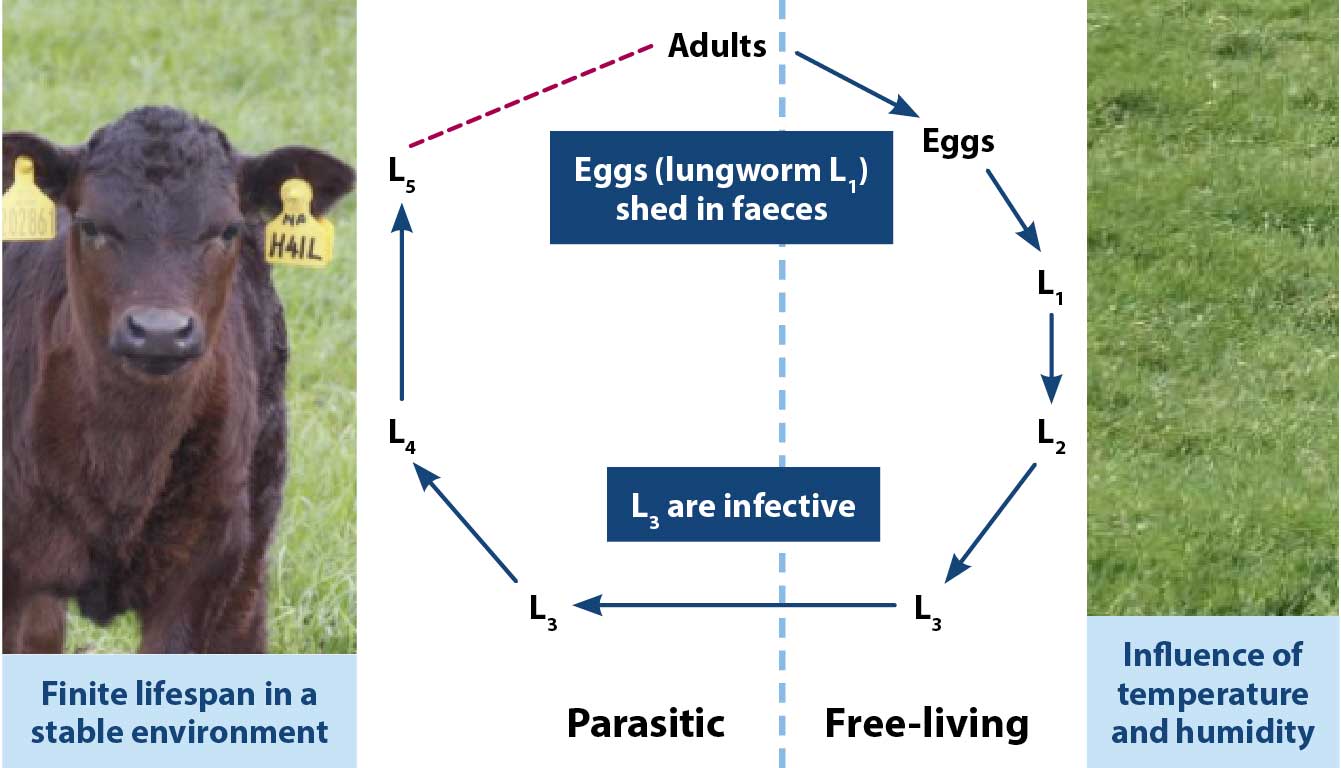
First-stage and second-stage larvae (L1 and L2, respectively) feed on faecal bacteria, while L3 retain the cuticle of the L2 as a protective sheath against desiccation, but cannot feed. L3 migrate on to herbage and are the infective larval stage. Once ingested by the host, the protective sheath is lost and further moults occur to the fourth larval stage (L4), before reaching the adult stage in their predilection site in the gastrointestinal tract or lungs.
D viviparus lungworms also have migratory life cycles, whereby newly acquired L3 and L4 move from the gut to the alveoli of the lungs via lymphatics and blood, while eggs shed by adult females in the large airways are coughed up, swallowed, hatch and are excreted in the faeces as L1.
The rates of egg hatching (gastrointestinal roundworms) and larval development – and larval survival – are influenced by temperature and humidity, respectively. Egg hatching and larval development only occur when temperatures in their environmental biomes are higher than 10°C, being optimal at about 20°C. Larvae develop faster at higher temperatures, but need more energy for metabolism and, therefore, have high mortality rates.
L3 are killed by desiccation, but may be protected during periods of hot and dry weather within their faecal biomes, or by moisture retained within dense pasture swords.
The burden of gastrointestinal roundworm L3 on pasture is determined by previous levels of egg shedding, and influences of temperature and humidity.
Overwintered L3 are the primary source of infection to calves turned out in spring. The size of this challenge is determined by the timing of grazing by cattle during the previous year, their burdens and winter weather conditions, in particular those giving rise to larval desiccation and death.
After 17 days – referred to as the prepatent period – overwintered L3 ingested by naive calves reach adulthood, mate and commence egg shedding. As late spring and summer temperatures increase – and given adequate moisture in the faecal biomes – the rate of development of these eggs to L3 increases, potentially to take just a few weeks. Rainfall is required to release the L3 from the faeces and for their migration on to herbage.
Each adult female O ostertagi sheds hundreds of eggs per day; therefore, small numbers of overwintered L3 can rapidly give rise to high levels of challenge. In the case of dairy or autumn-born beef suckler calves during their first grazing season, this can give rise to production-limiting disease within a few months of turnout.
In the case of spring-born beef suckler calves, most overwintered L3 are ingested by their immune dams and do not complete their development to patency. Consequently, these calves are not exposed to sufficient challenge during their first grazing season to develop protective immunity and remain naive when turned out for a second grazing season during the following spring.
Dry weather conditions throughout the summer, followed by rainfall in the autumn, can result in the en masse release of L3 that had developed and been protected within the faeces.
Adult female nematodes can only produce a certain number of eggs during their finite lifespans, while eggs shed when temperatures are lower than 10°C – or when conditions are hot and dry – will not hatch or survive.
O ostertagi has adapted to undergo developmental arrest as hypobiotic early fourth-stage larvae (EL4) in the abomasal glands during those cold, or hot and dry, periods that are not conducive to egg hatching or larval survival. In temperate climates, the onset of hypobiosis usually occurs during the late autumn, while EL4 emerge from the abomasal glands en masse in the spring – causing severe diarrhoea, and fluid and electrolyte imbalance; referred to as type-two ostertagiosis. This disease is most commonly seen following the aforementioned dry summers and wet autumns.
The epidemiology of D viviparus is much less clear due to additional influences on L3 availability, such as:
Therefore, lungworm outbreaks can be unpredictable and sometimes occur on pastures that have not been grazed by cattle in preceding years, or on recently ploughed reseeded fields.
The established principles of gastrointestinal roundworm and lungworm control are to reduce host exposure to L3 to levels that will allow for the development of protective immunity, while enabling parasitic stage burdens to be manageable.
This requires the integration of evasive host management, by grazing animals on fields that are predicted to harbour low levels of L3 challenge; and anthelmintic drug treatments to interrupt the parasites’ life histories, by suppressing egg or L1 shedding by infected cattle, thereby reducing the potential for larval development and buildup of pasture L3 contamination.
The timing of these approaches is governed by the annual production cycle of the host, resulting in the seasonal presence of naive or less immunologically capable animals, and environmental conditions favouring larval development and survival.
The most sustainable nematode control programmes for individual cattle herds are based on the application of understanding of the farming system and environment, and inferences on the relationship between pasture contamination, the availability of L3 on pasture and the accumulation of infection in animals.
However, most cattle producers administer anthelmintic drugs with the understandable, but irrational, goal of eradicating the parasites from their animals, frequently relying on a convenient-to-apply single group or active (Figure 2).
Questions must be asked concerning the responsibility of this approach, with reference to gaps in the acquisition of protective immunity, and parasite adaptation to changing patterns of farm management, climate and anthelmintic drug use.

Most cattle producers around the world use anthelmintic drugs rather than grazing management, or integrated control to manage gastrointestinal roundworms and lungworms.
This is understandable, because anthelmintic drugs are relatively inexpensive and convenient to use, while the application of evasive management strategies is complicated and may not always fit well with other farming activities.
Conversely, pharmaceutical control regimes are perceived to be straightforward due to the availability of macrocyclic lactone drugs with long persistence against reinfection with O ostertagi and D viviparus.
This strategy is highly effective, which, in itself, gives rise to challenges. Disease outbreaks have been reported, arising as a consequence of persistence of activity of macrocylic lactone drugs against reinfection with O ostertagi, but not Cooperia oncophora or Nematodirus helvetianus – creating opportunities for the establishment of the latter species in the absence of competitive influences of the former.
Use of persistent anthelmintic drugs in set stocked calves prevents the establishment of overwintered L3 acquired during the period of persistence, while suppressing egg output and preventing pasture L3 contamination once the persistent activity has ended – thereby eliminating exposure to O ostertagi altogether. This scenario prevents the acquisition of protective immunity during the calves’ first grazing season away from their dams, and necessitates control measures in subsequent grazing seasons to prevent disease outbreaks and production loss in older calves and adult cattle. This becomes expensive and possibly increases selection pressures for the emergence of anthelmintic resistance.
The clinical incidence of lungworm in older calves and adult cattle in the UK and Ireland has increased recently.
The use of persistent anthelmintic drugs in set stocked calves may not prevent exposure to lungworm L3 that may emerge from the soil, or be wind-borne during the latter part of the grazing season once the drug persistence has ended. This exposure may be sufficient to evoke host responses causing disease, but insufficient to maintain short-lived acquired immunity; therefore, cattle may remain susceptible to lungworm in subsequent grazing seasons.
Lungworm infection in previously exposed older cattle – sometimes referred to as reinfection syndrome – can result in clinical signs of respiratory disease, dramatically reduced milk yields, weight loss and sometimes death, depending on the levels of challenge and of host immunity.
A highly effective lungworm vaccine was developed in the 1950s at the University of Glasgow Veterinary School as the first vaccine in the world against a parasitic infection. Today, the unpredictable nature of D viviparus – and potential severity of disease – provides a compelling argument to reverse the current underutilisation of lungworm vaccination and develop novel strategies for its use.
Gamma-irradiated D viviparus L3 are administered orally to mimic natural infection without completing their life cycle and causing clinical disease. Calves older than two months of age are given two doses, separated by four weeks, with the second dose given before turnout to grass. Care is needed to avoid killing the vaccine L3 or preventing boosting of immunity by the use of persistent anthelmintic drugs.
It has been suggested that pulse-release oxfendazole boluses may provide opportunities for exposure to D viviparus between pulses sufficient to boost lungworm immunity, but little empirical evidence exists in support of this strategy.
Parasitic nematodes have high reproductive rates, large genomes with large numbers of genes, and high levels of polymorphism.
For example, the Haemonchus contortus chromosomal scale genome assembly is about 280 millions of base pairs with about 22,000 protein coding genes, while a single female may shed more than 4,000 eggs per day when conditions are suitable for their development.
Meanwhile, the genome assembly of Teladorsagia circumcincta – which is closely phylogenetically related to O ostertagi – is less refined, but known to be larger and more polymorphic.
Therefore, the concept of sustainability with regards to gastrointestinal roundworm and lungworm control needs to consider the inevitability of parasite adaptation in response to both favourable and unfavourable conditions. These may be afforded by changing climatic, human lifestyle or livestock management environmental effects on free-living stages, and the exposure of parasitic stages to anthelmintic drugs.
We have described several examples of evolutionary or epigenetic parasitic nematode adaptation to environmental change, and reported resistance to each of the single-active, broad-spectrum anthelmintic groups in gastrointestinal roundworms of Scottish sheep. We have investigated the factors that may have given rise to their emergence.
Globally, benzimidazole, imidazothiazole and macrocyclic lactone anthelmintic resistance is widespread in most major trichostrongyle species infecting small ruminants, while multigeneric anthelmintic resistance to the amino acetyl nitrate derivative drug, monepantel, is emerging.
Salicylanilide resistance is widespread in small ruminant H contortus, resistance to benzimidazole and macrocyclic lactone drugs has been reported in gastrointestinal roundworm species infecting cattle, and resistance to most anthelmintics is suspected in various species of human helminth parasites.
Therefore, a need exists for practical cattle gastrointestinal roundworm and lungworm control strategies, integrating grazing management and use of anthelmintic drugs in specific agricultural and environmental contexts – drawing on expertise gained from small ruminant work to incorporate anthelmintic resistance mitigation, and explore the exploitation of host-genetic adaption, use of natural xenobiotics and vaccine development.