22 May 2023
George Lindley, Hannah Fitzsimmonds and Stephanie Prior look at understanding these minerals – as well as how to diagnose, treat and prevent deficiencies – in cattle health.

Image: © Susan Hutton / Adobe Stock
Trace elements are an important cause of production losses in the UK cattle industry, with levels influenced by farm location and geography, soil health and rationing.
This article will discuss the trace elements iodine, selenium, vitamin E, copper and cobalt, which may represent those most clinically relevant for practitioners within the UK. Nonetheless, it should be considered that, on occasion, other less appreciated trace elements, including iron and copper, may also have meaningful consequences for cattle health and fertility.
Many impacts of deficiency and toxicity of trace elements exist, and their interactions are complex. Testing or screening for trace elements can be challenging, with some diagnostic tests more accurate than others, but more invasive. If unsure, helpful advice regarding sample collection can usually be obtained from any diagnostic laboratory and this may be advisable prior to the collection of any specimens.
Iodine is an integral constituent of the thyroid hormones and, therefore, necessary for both growth and development.
Deficiencies in iodine can lead to hypothyroidism, goitre, delayed development, abortions, weakness and increases in mortality (Graham, 1991). Iodine deficiency-related fetal death may occur at any stage of gestation and calves may be born hairless, weak or dead.
Iodine deficiency can be primary due to a lack of iodine in the diet or secondary due to selenium deficiency, or the presence of goitrogens. Selenium plays an important role within thyroid hormone metabolism (Arthur et al, 1996), and should be investigated where iodine deficiencies are suspected.
Goitrogens represent naturally occurring substances that naturally interfere with the uptake of iodine in the thyroid gland. Thiocyanate is an important dietary goitrogen that is formed during the metabolism of glucosinolates, present within plants of the Brassica family. Therefore, animals fed on diets including forages such as kale, turnips, white clover or other Brassicas, may need additional iodine supplementation to counteract the goitrogenic effects (Ensley, 2020).
Clinical diagnosis of iodine toxicity can be made by the presence of a goitre on clinical examination. In stillborn calves, removing and weighing the thyroid gland can give an indication of the issue. Thyroid glands weighing more than 14g would be considered suggestive of iodine deficiency (Table 1), which may be confirmed by histopathological examination of the gland (Andrews et al, 2003). Care should be taken when blood sampling for plasma inorganic iodine, as recent intakes can lead to falsely high results.
| Table 1. A summary of the key trace elements required by cattle (modified from Cockcroft, 2015) | ||||
|---|---|---|---|---|
| Trace element | Feed rate per kg | Testing | Clinical implications | Treatment |
| Iodine | 0.5mg-2mg | Thyroid gland weight and histopathology |
↑ Respiratory distress, weight loss ↓ Fetal death |
Dietary supplementation or slow-release bolus |
| Selenium Vitamin E |
0.12mg 25iu |
Serum glutathione perioxidase Liver biopsies Supplementation trial |
↑ Respiratory distress ↓ White muscle dystrophy (congenital or degenerative), sudden death in youngstock, retained fetal membranes, fertility issues |
Dietary supplementation Slow-release bolus Injectable alpha-tocopherol acetate and sodium selenate |
| Copper | 12mg | Liver biopsies Liver or kidney postmortem samples |
↑ Jaundice anaemia, death ↓ Decreased growth rates, reduced immune function |
Dietary supplementation or slow release bolus |
| Cobalt | 0.3mg | Serum vitamin B12 Liver biopsies Supplementation trial |
↓ Ill thrift, anorexia, reduced weight gain, reduced immunity, coarse coat |
Dietary supplementation Slow release bolus Injectable cobalamin |
| Manganese | 40mg | Supplementation trial | ↓ Congenital joint laxity and dwarfism | Dietary supplementation |
| ↑ = Excess, ↓ = Deficiency | ||||
Iodine toxicity may show as hyperthermia, persistent cough, naso-ocular discharge, tachycardia and weight loss. Necroscopy findings are normally related to the respiratory tract, including tracheitis, bronchopneumonia and pleurisy (Paulíková et al, 2002).
The antioxidant roles of selenium and vitamin E are closely linked. Selenium is associated with many selenoproteins, such as glutathione perioxidase (GSPx), which act to neutralise reactive oxygen species (ROS), including free radicals and peroxides (Kang et al, 2020). Vitamin E plays a complementary role as it acts to scavenge ROS, which may have a selenoprotein-sparing effect. For this reason, in some instances, excess of one may compensate for deficiencies in the other.
Selenium deficiency can present in different ways depending on age. Calves deficient in selenium or in vitamin E can present with white muscle disease (WMD).
WMD may occur as a congenital dystrophy, which may be caused by low intakes of selenium/vitamin E during late gestation (McDowell et al, 1996). Alternatively, a delayed myopathy may be seen. These cases are most common at spring turnout, due to the effect of increased exercise, which increases oxidative stress. Inadequate vitamin E intakes may also contribute to this manifestation (Suttle, 2010).
Adult cattle with selenium or vitamin E deficiency have higher incidence of retained placentas, and are predisposed to metritis, mastitis and cystic ovaries. This is, in part, due to the gradual decrease of alpha-tocopherol during pregnancy – reaching its lowest level at calving – with this decline most recorded in high-yielding dairy cattle.
Dairy cattle that go on to develop left displaced abomasums have high incidence rates of hypovitaminosis E seven days post-calving in comparison to cows that do not (Haga et al, 2021). Nonetheless, in herds with a high incidence of periparturient disease, investigation of trace element status should be considered a single component of a complete transition cow evaluation.
In bulls, deficiency in vitamin E and selenium is associated with reduced fertility – primarily due to negative effects with spermatozoon synthesis (Mehdi et al, 2016).
Toxicity is very rare, but in acute form presents as respiratory distress and rapid deterioration. Chronic selenosis or “alkali disease” may be the result of sustained consumption of seleniferous feed for weeks or months, and may result in emaciation, lameness, blindness and liver cirrhosis (James and Shupe, 1984).
Diagnosis of selenium deficiency is most commonly achieved by measuring GSPx activity within whole blood or serum samples. Whole blood samples provide an indication of red blood cell GSPx concentrations.
During erythropoiesis, GSPx is incorporated into red blood cells four to six weeks prior to their release into the blood stream (Radostits et al, 2007). As a result, whole blood samples are a relatively historic measure, while plasma or serum GSPx is a more contemporary measure (Villar et al, 2002).
Treatment of selenium deficiencies ranges from dietary supplementation, slow-release bolus (usually in combination with other trace elements), or in cases of concern for WMD on farm, combination injectables containing alpha-tocopherol acetate and sodium selenate can be administered.
Copper deficiency is typically seen in young animals at pasture presenting with low growth rates. Copper is important in a functional immune system, as well as for growth and development. Copper-deficient animals often have thin skin, and their coat may lose its colour due to depigmentation. This can lead to the pathognomonic sign of “spectacles” around the animal’s eyes.
Fertility may be affected in animals with copper deficiency, such as a delay to cyclicity in maiden heifers. Chronic copper deficiency may lead to a reduction in immune function as well as anaemia.
Although copper deficiency can be seen in animals without sufficient intakes of dietary copper, it is more common to see the signs in animals with a diet high in copper antagonists such as molybdenum. In the rumen, molybdenum can bind to copper, sulphur and iron, leading to the formation of thiomolybdates, which can be absorbed into the bloodstream (Gould and Kendall, 2011). Thiomolybdate toxicity can occur, which appears – with regards to clinical signs – to be very similar to copper deficiency.
Acute copper toxicity can occur when a sudden intake of large amounts of copper occurs – for example, accidental parenteral supplementation. However, chronic toxicity is much more common.
Chronic copper toxicity occurs when copper intakes are increased over a period of weeks to months, although the clinical signs may only become apparent towards the end stage of damage. This can be caused by long-term over-supplementation of dietary copper, which may occur when supplementation is concomitantly provided in many forms (for example, additional minerals in hard feed, rumen bolus and so on).
Any copper over the animals’ requirements is normally accumulated in the liver. An acute event can lead to the release of large amounts of copper into the bloodstream, leading to intravascular haemolysis, jaundice and death.
Liver biopsies can be useful in determining copper levels; however, copper isn’t stored uniformly throughout the liver, so this is most useful as a herd screening tool. Poor correlation exists between serum and liver concentration (Johnston et al, 2014).
The role of trace element cobalt is for the formation of vitamin B12, which in broad terms forms energy from ruminal fermentation.
Cobalt is the limiting factor for vitamin B12 production by ruminants; therefore, clinical signs attributed to vitamin B12 deficiency can be viewed as a cobalt deficiency.
Up until the rumen is fully developed at six to eight weeks, calves rely on dietary vitamin B12 to fulfil their requirements, after which rumen microflora will take over production in the presence of adequate cobalt.
Clinical signs of cobalt deficiency can be quite non-descript, with general ill-thrift, anorexia and poor weight gain being common. Lower immunity, including higher susceptibility to parasites, is also seen (Chamberlain and Wilkinson, 1996). Coats can become sparse, although this is more common in sheep with cobalt deficiencies.
As the deficiency becomes more chronic, anaemia and hepatic lipidosis develop, furthering weight loss and inappetence. Younger cattle are more prone to displaying clinical signs of deficiencies due to their proportionally lower reserves of vitamin B12 in the liver.
Cases of toxicity are rare. Difficulties can be seen in cattle fed high concentrate diets, such as finishing cattle, due to poor cobalt availability in grains (González-Montaña et al, 2020). Grazed cattle may be impacted by land with soil pH more than 6.5, as uptake of cobalt into the grass may be poor.
Testing for cobalt deficiency uses measuring levels of vitamin B12 in serum or liver samples. This testing is not reliable, in part due to variability of cobalamin analogues interfering with interpretation.
Treatment of a group suspect of deficiencies and monitoring results is commonplace to confirm suspicion. Options for this include slow-release boluses of cobalt, or use of injectable cobalamin. A full review of the diet is important if an issue is suspected.
Most commonly, manganese deficiency has been implicated as a possible contributing factor to congenital joint laxity and dwarfism (CJLD; Figures 1 and 2) in spring-calving beef suckler herds (Hidroglou et al, 1990; Valero et al, 1990).

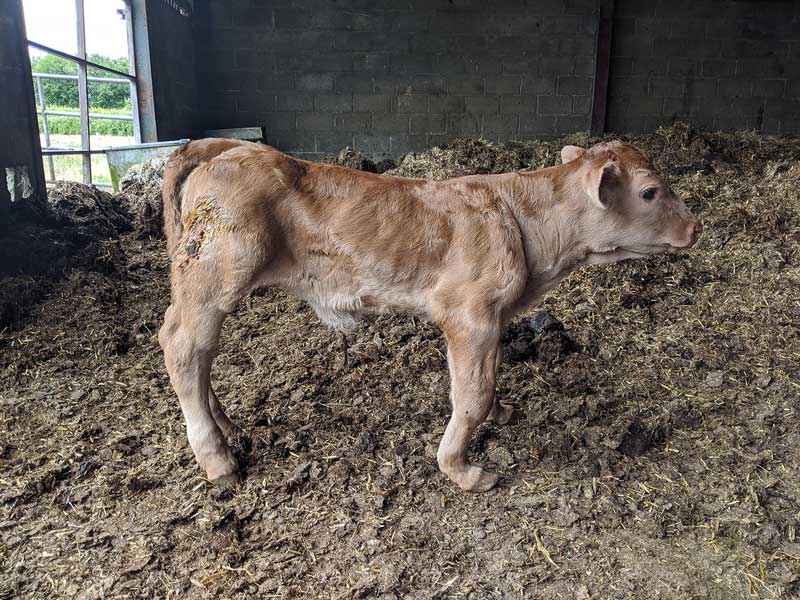
The precise aetiology of CJLD remains unknown and the cause may not be due to an absolute deficiency of dietary manganese, but rather a relative deficiency in manganese bioavailability, due to intakes of soil or mineral antagonists, or due to the effects of ruminal fermentation (Hidirglou et al, 1990; Mclaren et al, 2007; White et al, 2010).
Diagnosis is difficult. Low liver tissue manganese concentrations may be suggestive of deficiency, but if collected, postmortem samples must be taken from calves that have not suckled, as concentrations rise rapidly afterwards.
Most commonly, a suggestive diagnosis is made following resolution of the issues after dietary change. Nonetheless, other teratogenic agents – such as bovine viral diarrhoea, Schmallenberg virus or bluetongue virus, as well as mycotoxins and lupine toxicity – should also be considered.
Risk factors associated with the occurrence of CJLD include the feeding of grass-silage only during mid-gestation, and the exposure of pregnant cows to drought conditions at pasture during the final five months of gestation. Prevention in these circumstances is focused on the addition of supplemental grain or hay, and ensuring a high-quality, nutritionally complete diet is provided at pasture and during housing (Valero et al, 1990).
While manganese supplementation is commonly recommended, its effectiveness remains unproven (Underwood and Suttle, 2001), and in the authors’ experience in herds where the proportion of fed grass silage is reduced, additional supplementation has had no additional benefit. If desired, the benefits of supplementation may be identified by treating a group of animals and comparing their performance in comparison to untreated counterparts.
Many clinical signs of trace element deficiencies or toxicities exist, with some easier to recognise and more pathognomic than others.
Testing for trace element deficiency should be performed as a component of investigations into the wider context of health and fertility on an individual farm. In these circumstances, an informed understanding of the limitations and diagnostic testing methods is required prior to sample collection.
The authors’ group at the RVC is looking to recruit farms and their veterinary practices for a PhD study, entitled “Assessment of current colostrum feeding practices and extent of passive transfer in dairy herds in the UK”.
It would like to use serum total protein data, which is usually collected by vets, to evaluate if calves have adequate immunity from the colostrum they were provided, alongside a short questionnaire.
Its aim is to determine an estimate of the amount of failure of passive transfer within UK dairy herds, as well as to understand how dairy farmers are currently feeding colostrum. The results will allow benchmarking of herds to be performed on a national level, which it hopes to be able to report to enrolled farmers and practices by the end of 2023.
The success of the project is somewhat dependent on recruitment of a large number of calves, farms and practices, and the group is enthusiastic about discussing the project with practices and farmers who might be keen to get involved. If the project is something that you might consider participating in, or if you would like more information, contact George via [email protected]