30 Jun 2021
Updates on cattle endoparasites
Hany Elsheikha reviews current and expected prevalence and treatment protocols in herds, including resistance considerations.

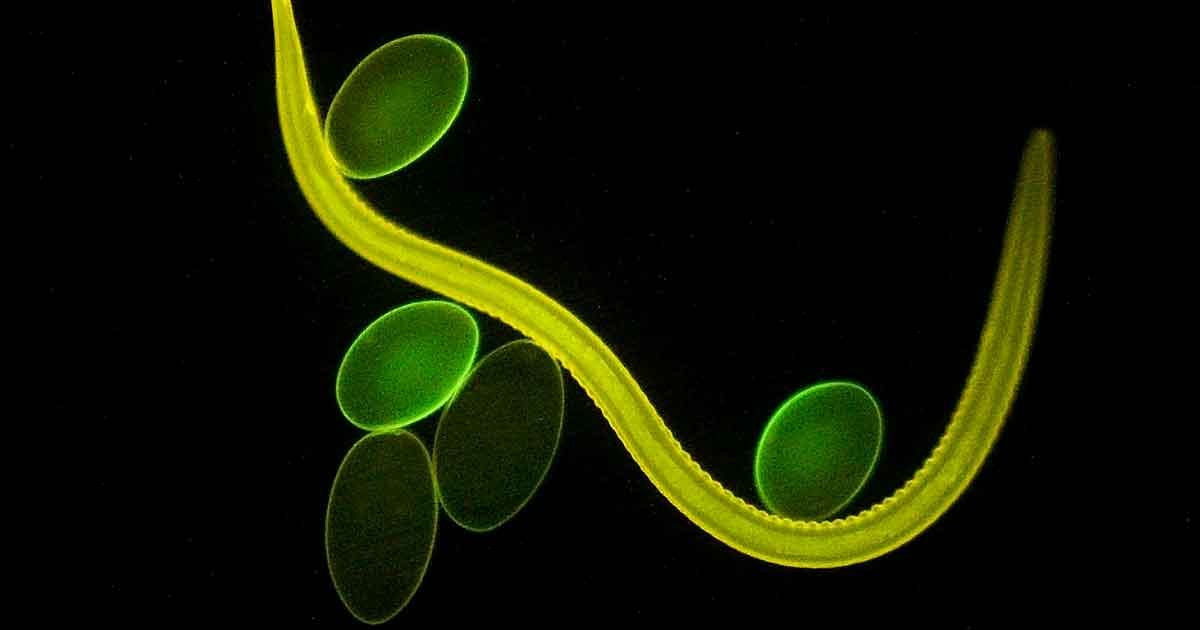
Figure 1. Haemonchus contortus adult and eggs. Image: © Oregon State University / CC BY-SA 2.0
The commercial cattle production has increased in recent decades, with further growth projected to maintain the food security of the world’s growing population.
Endoparasites, including worms/helminths and protozoa, are the leading cause of serious parasite-related gastrointestinal infections in cattle worldwide. Therefore, efficient control of endoparasites is important to maintain cattle health and welfare, support global food security efforts and, potentially, public health.
Despite the many chemotherapeutic options and the considerable effort that has been spent on developing parasite control programmes, endoparasite control remains a challenge in nearly all parts of the world. The situation is further aggravated by the increasing prevalence of resistance to anthelmintics among many helminths. The development of new anthelmintics is expensive and can take decades.
Additionally, no licensed vaccines exist for parasite control in cattle, except the vaccine used in the prevention of lungworms and the recently developed vaccine against the barber’s pole worms. These challenges put the cattle industry under threat. Therefore, the effectiveness of existing anthelmintics should be maintained by designing integrated parasite control approaches, combining the sensible use of anthelmintics and good grazing practices.
While farm animal veterinarians play a key role in implementing parasite control programmes, parasitologists can bring significant and novel insights into the formulation and success of these programmes.
Cattle can be infected by a large number of parasitic worms/helminths, including many species of nematodes (roundworms), trematodes (flukes) and cestodes (tapeworms).
Cattle can also be infected by a number of protozoa, such as Eimeria species, Giardia species and Cryptosporidium species. All these parasites are transmitted via the faecal‑oral route and associated with gastrointestinal manifestations.
The zoonotic potential and public health risk of some of these parasites – such as Fasciola species, Giardia species and Cryptosporidium species – make the better understanding of cattle endoparasites a key focus of veterinary and human parasite control programmes.
The aim of this article is to discuss key aspects of infections caused by endoparasites in cattle.
Nematodes (roundworms)
Gastrointestinal nematodes
Cattle gastrointestinal roundworms include species that live in the abomasum. These are Haemonchus placei, Ostertagia ostertagi and Trichostrongylus axei. Haemonchus contortus (Figure 1) mainly infects sheep, but can be also found in cattle.
Intestinal roundworms that inhabit the small intestine of cattle include Cooperia oncophora, Cooperia punctata, Cooperia pectinata, Trichostrongylus colubriformis, Trichostrongylus vitrinus, Nematodirus helvetianus, Bunostomum phlebotomum and Capillaria bovis.
Large intestinal roundworms include Oesophagostomum radiatum and Trichuris discolor.
The life cycles of these roundworms are direct and follow a similar pattern (Elsheikha and Khan, 2011).
Cattle acquire infection orally from ingestion of contaminated pasture containing infective third‑stage larvae. Gastrointestinal nematodes cost the cattle industry in North America more than US$2 billion (£1.41 billion) annually (Stromberg and Gasbarre, 2006).
Anthelmintic drugs that are available for the treatment of gastrointestinal roundworm infections belong to five different chemical classes and include benzimidazoles, levamisole, macrocyclic lactones, aminoacetonitrile derivatives (monepantel) and spiroindoles (derquantel). These anthelmintics are effective against many species of gastrointestinal nematodes and lungworms. Some of these products also have activity against liver flukes.
Macrocyclic lactones are often referred to as endectocides due to their additional activity against external parasites. Other anthelmintic products have narrow spectrum and are only effective against particular parasite species.
Anthelmintic drugs should be chosen to target the developmental stage of the worm (whether adult or juvenile form) highly likely to be involved in the infection, or that has already been identified using laboratory diagnostic methods, such as faecal worm egg count.
In an effort to survive during adverse climatic conditions, many roundworms undergo a process known as hypobiosis, where the ingested third‑stage larvae halt their development at the early fourth stage. These dormant larvae resume their growth the next grazing season, resulting in clinical disease in older cattle (for example, type‑two ostertagiosis). This phenomenon of hypobiosis allows the cattle to keep the parasite burden in check.
Arrested parasite development also reduces the level of pasture contamination to a level that does not put the grazing cattle at a risk of developing clinical disease.
Not all anthelmintic products have potency against hypobiotic larvae.
Lungworms
Dictyocaulus viviparus is an important roundworm infecting the lung of cattle in temperate regions. It causes a disease known as parasitic bronchitis, husk or dictyocaulosis, which commonly affects first-season grazing calves; however, infection has also been reported in adult cows.
Dictyocaulosis can cause reduction in weight gain and milk production, and mortality that can reach 20 per cent (Holzhauer et al, 2011).
The following factors should be avoided because they increase cattle exposure to D viviparus:
- introduction of new animals to the herd
- heavy use of anthelmintics
- presence of water bodies on pasture
The diagnosis of D viviparus infection is based on the recovery of larvae from faeces using the Baermann method or by using immune assays, such as ELISA.
Dictyocaulosis can be treated using levamisole, benzimidazoles or macrocyclic lactones; are all effective against lungworm infection. Early and timely intervention before the onset of clinical signs can prevent the disease and allow the establishment of protective immunity.
A lungworm‑effective vaccine exists and is made up of irradiated third-stage (infective) larvae. Vaccination against husk can be a vital component of any lungworm control programme. However, this vaccine does not produce persistent protection without subsequent natural infections or annual booster vaccination.
Trematodes (flukes)
Liver flukes (Fasciola species)
Fasciolosis, a widespread parasitic infection of ruminants, is caused by liver flukes of the genus Fasciola. This disease causes a significant impact on the productivity of food-producing animals, including cattle.
The increasing prevalence of liver flukes has been attributed to the increased animal movements, changing farming practices and climate change.
Current diagnostic tests include faecal examination for the detection of fluke eggs and serological assays for detection of the presence of antibodies to Fasciola.
Fasciola species also infect a broad range of mammalian hosts and can spread to the human sphere; millions of people are at risk of infection (Sabourin et al, 2018). Therefore, effective parasite management strategies are needed to control this disease, to protect both animal and human health.
Cattle often suffer from chronic fasciolosis; therefore, treatment should target adult flukes to save the animal productivity and to reduce pasture contamination.
Control of Fasciola infection relies on the use of flukicidal drugs. A wide selection of such products are on the market, including oxyclozanide, clorsulon, closantel, nitroxynil and rafoxanide. Triclabendazole is widely used because it is effective against adult and juvenile stages of liver flukes; however, triclabendazole resistance has been reported (Fairweather et al, 2020).
Some products available to treat adult flukes are licensed for use in lactating dairy cattle, such as those containing albendazole (60‑hour milk withdrawal time) and oxyclozanide (72‑hour milk withdrawal time).
Pasture management, including fencing off or draining high-risk areas, can reduce exposure of cattle to liver fluke risk.
Ruminal flukes (paramphistomes)
Although paramphistomid infections have been traditionally limited to tropical and subtropical regions, the prevalence of these ruminal flukes in ruminants in Europe has been progressively increasing over the past 10 to 15 years in the UK and several European countries (Taylor, 2012; Gordon et al, 2013).
This can be attributed to climate change, importation of infected animals, improvement in the diagnostic capabilities, the use of anthelmintics with limited efficacy against ruminal flukes, and the adaptation of the ruminal flukes to their intermediate host, the Galba truncatula snail.
The primary paramphistomid species detected in Europe is Calicophoron daubneyi. Paramphistomum leydeni has been detected infrequently in England, Ireland and the Netherlands.
Cattle infected by ruminal flukes are more likely to be infected by F hepatica, which can be attributed to the fact both parasites share the same intermediate host and require similar environmental conditions to complete their development. However, whether the presence of paramphistomes on a farm influences the risk of infection and consequent losses due to F hepatica remains unknown.
Paramphistomid infections can compromise the animal productivity and growth rate. Immature flukes developing in the small intestine are the cause of the clinical disease.
Diagnosis of paramphistome infection depends on the detection of the paramphistomid eggs in ruminant faeces. At postmortem examination, adult flukes can be seen attached to the mucosa of the first two compartments of cattle stomach.
No anthelminthic product is currently approved for treatment of ruminal flukes in the UK. The traditional anthelmintics are ineffective against ruminal flukes.
A single dose of 15mg/kg of oxyclozanide or 10mg/kg of closantel has good efficacy against ruminal flukes in cattle (García-Dios et al, 2020). Oxyclozanide, which is used for the treatment of adult F hepatica infection, is also effective against immature and mature stages of ruminal flukes (Paraud et al, 2009).
The higher prevalence of ruminal flukes in the western regions of Great Britain has been attributed to high rainfall or access of cattle to watercourses (Fenemore et al, 2021). Therefore, strategic treatments of ruminal flukes using flukicides should be supported by complementary control measures – such as avoidance or reducing access of cattle to watercourses, and drainage of snail habitats – for reducing pasture contamination with fluke eggs.
Cestodes (tapeworms)
Moniezia, Avitellina and Thysanosoma tapeworms inhabit the small intestine of ruminants, and have a worldwide distribution. Heavy infection can lead to intestinal obstruction, particularly in young ruminants. Several benzimidazole anthelmintic products are effective against tapeworms (www.cattleparasites.org.uk).
What is anthelmintic resistance and how does it happen?
The development of resistance to anthelmintics by many parasitic roundworms is a growing problem worldwide.
Although anthelmintic resistance (AR) is widespread in parasites of sheep, there are reports from a number of countries indicating that AR also occurs in cattle gastrointestinal nematodes – including H contortus, H placei, C punctata, C oncophora, Cooperia spatulata and O ostertagi.
AR develops when a drug loses its activity against one or more species of parasites that was previously susceptible. In general, AR develops due to many factors – especially underdosing, frequent treatments and low refugia (Elsheikha and Khan, 2011; Sutherland and Leathwick, 2011). Therefore, the frequency of treatment is a potent source of selection pressure that occurs whenever a drug is often used.
Resistant worms pass on their genetic attributes to the next generation, thereby amplifying the frequency of their resistant genes in the parasite population.
The problem of resistance to anthelmintics is multifaceted. The genetic basis, the modes of inheritance of resistance and the pace at which AR to a particular wormer evolves are largely undefined, and vary significantly among the various chemical classes of anthelmintics.
Given these challenges and the limited alternative options to worm control, regular evaluation of the extent and impact of AR at the farm level, using a faecal worm egg count reduction test, is a necessary measure to achieve effective parasite control regimens.
How can AR be managed?
Strict measures should be taken to tackle the growing threat of AR because it threatens the sustainability of efficient farming practices (McKellar and Jackson, 2004; Kaplan, 2020). This should be achieved by adopting an integrated parasite management approach and reducing the sole dependence on anthelmintic drugs.
While the strategic use of antiparasitic drugs is valuable in managing adverse impacts of parasitism on animals, grazing management tools – such as provision of clean pastures, alternate grazing by different animal species, alternate grazing by immunologically resistant hosts of the same species, maintaining refugia, and monitoring of parasite burden – can also support the effectiveness of anthelmintic drugs.
Sound pasture management reduces the need for anthelmintics, and minimises reinfection by preventing the contamination of pastures and the buildup of infective worm eggs or larvae on pasture.
Farmers must be well-informed about the most appropriate use of wormers and ensure they are able to follow evidence‑based guidelines on the effective use of cattle wormers (www.cattleparasites.org.uk). Individuals who administer anthelmintics must read the product leaflet and summary of product characteristics, and ensure the prescribed dose by weighing the animals or using a weight band.
It is important to base the treatment programme on evidence of existing infection. Faecal egg counts, together with monitoring animals’ weight and condition, can give a general idea of whether treatment is needed.
Another solution to the problem of resistance to a single anthelmintic treatment is the combined administration of anthelmintics with non‑overlapping/dissimilar mechanisms of action. This empirical concept of combination treatment has been sought as an alternative strategy to manage resistant gastrointestinal roundworms (Geary et al, 2012).
A recent study found that four-year co-administration of moxidectin and levamisole in lambs with multiresistant gastrointestinal roundworms resulted in a significantly better efficacy (87 per cent) than that of moxidectin (42 per cent) or levamisole (69 per cent) administered separately (Luque et al, 2021). However, this combinational treatment approach still requires a lot of validation and optimisation studies, and may increase the complexity of parasite control regimens.
Refugia (those in refuge) refers to the parasite’s subpopulation that is not exposed to anthelmintic treatment. Refugia includes:
- developmental stages outside the host on pasture
- parasites in untreated animals
- parasitic stages within the animals, but are not exposed to the treatment (for example, hypobiotic larvae)
The parasites in refugia provide a source of drug-susceptible worms with susceptible gene alleles to mate with drug-resistant worms, which will result in more offspring with unselected genes. Any increase in the refugia will lead to a reduction in the rate of AR development and vice versa.
Vaccination has been a great tool in the control of many infectious diseases, but this is not the case when it comes to parasitic diseases. Despite the significant advancements in the understanding of the immunobiology of helminth infections, this improved knowledge has not made the prospect of helminth vaccines more achievable.
Fortunately, a vaccine is available for lungworm control. Also, a vaccine exists for the barber’s pole worm, H contortus, which is marketed in Australia and South Africa. It can be used to control haemonchosis in sheep and can work against H placei in calves (Bassetto et al, 2014), but this vaccine is not licensed for use in cattle. It can be particularly useful in situations where AR is widespread.
Natural products have provided a considerable resource for the discovery of novel substances with high anthelmintic activities. A previous study showed that cysteine proteinases derived from papaya latex have a significant anthelmintic activity against the abomasal nematode H contortus (Buttle et al, 2011). Another recent study showed that coriander oil and linalool combinations (Figure 2) confer a synergistic anthelmintic effect on the motility of infective larvae of five major gastrointestinal nematodes of ruminants (Helal et al, 2020).
Gastrointestinal protozoans
Cattle are susceptible to infection by a number of protozoa, which can have a major burden on the health and welfare of the infected animals, particularly young calves.
Various Eimeria species are found in the gastrointestinal tract of cattle, which become infected by ingesting sporulated oocysts via faecal-oral route while grazing contaminated pasture. Several species of Eimeria can infect cattle; however, Eimeria bovis and Eimeria zuernii are the leading cause of enteric disease, and can even kill young calves.
Eimeria organisms multiply rapidly and cause massive destruction in the intestinal mucosa, leading to malabsorption of nutrients, and watery or bloody diarrhoea, particularly in young animals. Intensive calf rearing, poor hygiene and stressful situations (such as transport, overcrowding and weaning) favour the multiplication of these protozoa.
Disease caused by Eimeria infection – known as coccidiosis – is associated with significant production losses because the affected animals exhibit loss of appetite, depression, dehydration, weight loss and retarded growth. Older cattle, unless seriously stressed or immunocompromised, are asymptomatic carriers and shed oocysts, causing more environmental contamination, and spread infection to other animals.
Eimeria oocysts can be detected using a faecal flotation test. Anticoccidial products (for example, diclazuril, toltrazuril) are often used for controlling coccidiosis in calves and can have high efficacy against pre-existing Eimeria infection.
Giardia duodenalis infection in ruminants is generally asymptomatic, but diarrhoea has been reported in young animals infected with this protozoan. Giardia cysts can be detected in faeces by using a double-centrifugation flotation test with zinc sulfate.
In addition to causing production losses in affected animals, G duodenalis has a public health impact due to its zoonotic potential.
Although no drugs are licensed for Giardia infection in ruminants, anthelmintic drugs such as albendazole and fenbendazole have been effective.
Several species of Cryptosporidium are reported in cattle; however, Cryptosporidium parvum is the most frequent and pathogenic Cryptosporidium species infecting calves. It is commonly associated with diarrhoea, loss of appetite, dehydration, reduced bodyweight and even death in preweaned calves.
Clinical cryptosporidiosis can potentially cost £130 per infected calf due to the reduction in weight gain (Shaw et al, 2020). C parvum is also zoonotic and can infect humans.
Diagnosis is achieved by using the double‑centrifugation Wisconsin method with sucrose solution where the oocysts appear refringent to light with a characteristic pink colour.
Oral halofuginone lactate is used to prevent or reduce diarrhoea due to C parvum in calves.
Conclusion
Maintaining efficient livestock production by preventing and treating endoparasitic infections is essential for meeting the increasing food demand of the growing world population.
Worm control in cattle rests on the use of anthelmintics, which will remain the most valuable tools in the treatment and control of worm infection. However, resistance problems can escalate with the excessive use of anthelmintics, which will compromise overall success of livestock worm control.
Cattle are also susceptible to infection by a number of protozoa, some of which can have a considerable medical significance in addition to their veterinary impact.
An effective endoparasite control programme should be designed to minimise parasite load in cattle, prevent contamination of the environment and interrupt the parasite’s transmission cycle, while maintaining the effectiveness of existing antiparasitic drugs.
Efforts should be expanded on the development of more innovative endoparasite control programmes that combine the sensible use of endoparasiticides and sound grazing management practices.
Conflict of interest
The author declares the article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. References to any drug do not imply their endorsement by the author or publisher.
It is important to read the most up-to-date product data sheets, as some restrictions on products – including meat and milk withdrawal periods – are prone to change as maximum residue limits change for individual products.
- Some drugs are used under the cascade.
