21 Oct 2025
Acute pancreatitis problems in dogs: what’s new? – Part one
Bryn Jones BVM, BVS, CertAVP, DipECVIM-CA, MRCVS sheds light on the research around this disease, its pathophysiology, complications and management, in the first of a two-part series.

Image: Eva / Adobe Stock
Canine acute pancreatitis (CAP) remains an important disease encountered regularly both in first opinion and referral practice.
In a survey of dog owners relating to some 40,000 dogs, 2% of dogs were reported to have suffered from pancreatitis at some stage in their life1.
This is, of course, not a new disease, but is a key area of ongoing research interest due to the very well-recognised challenges CAP poses in both its diagnosis and treatment.
Every vet will understand the pitfalls of discordant results and the difficulties in recommending appropriate treatment when the impact of the disease can vary so greatly with contrasting outcomes and timeframes.
This article is far from an exhaustive review on the subject, but highlights some of the data produced in the past five to six years, as it relates to our continued understanding of the pathophysiology, investigation, complications and, ultimately, management of CAP in all its forms.
Pathophysiology and causes
Often, the first question prompted by an owner upon diagnosis or suspicion of diagnosis in their dog is simply: “What has caused this?”
It can be thoroughly frustrating to receive probably the most common answer: “We just don’t know.”
It goes without saying that, as time has gone on, more diseases and toxins/drugs have been associated with the development of pancreatitis (no matter how tangentially). However, it still seems that most frequently the disease develops quickly and spontaneously, without a clear underlying trigger2.
At its heart, CAP is still a disease where local inflammation can quickly become systemic, aided substantially by the release of pancreatic enzymes into the systemic circulation. The release of digestive enzymes is understandably incredibly irritating (to put it mildly) to normal tissue and to the endothelium of most organs.
Pancreatitis occurs when acinar cell dysfunction and abnormal and early activation of trypsin and other enzymes occurs within the pancreas2,3. This overcomes the local protective processes and, eventually, the protective anti-proteases systemically4. An inflammatory amplification loop can eventually lead to systemic inflammatory response syndrome (SIRS) and multi-organ dysfunction5.
Trypsinogen-independent pathways also exist, though it is likely to be the final pathway still for most disease2. Oxidative stress appears to play an important role in the pathogenesis of CAP, too6. Pancreatic acinar inflammation progresses to local peritonitis and injury to the nearby gastrointestinal wall7.
Causes/risk factors
Pancreatitis can occur postoperatively, either by direct physical injury/manipulation of the organ or by surgery-associated inflammation of nearby tissues (especially the biliary tree).
Dogs undergoing cholecystectomy for gallbladder mucocele (GBM) have a 5.95% incidence of pancreatitis postoperatively, rising to 8.9% if the common bile duct (CBD) is catheterised8. In fact, flushing the CBD increases the overall risk of pancreatitis by 8.1 times, leading to a query over whether flushing should be performed at all8. The incidence in another cholecystectomy study (for a variety of reasons, but most commonly GBM) was even higher at 18.5%.
Dogs that develop pancreatitis post-cholecystectomy may have a much higher risk of death in hospital (four times the risk reported, though not confirmed on multivariable analysis)9. Pancreatitis can also be a sequela of hepatic and adrenal surgeries10,11.
Severe CAP was reported in a dog receiving meglumine antimoniate for leishmaniosis, building on older reports, and indeed a larger study showed a whopping 42% prevalence of pancreatitis for dogs receiving this drug, mostly during the treatment course12,13.
CAP has been reported in a dog following bee envenomation and subsequent hypersensitivity reaction three days later, which has also been recorded in people, too14. This is postulated to occur either secondary to anaphylactic hypotension and ischaemia, or instead from direct injury from the venom (especially via phospholipase).
While exogenous glucocorticoids have been implicated as a possible risk factor for CAP in the past, limited evidence exists for this concern. If glucocorticoids do potentially cause increases in pancreatic lipases, this is often not considered to be associated with clinically significant pancreatic injury2,15.
Pancreatitis continues to show association with diabetes mellitus, and is present in 10% to 11% of CAP and especially associated with ketoacidosis16-18. Diabetic dogs were 12.4 times more likely to develop CAP, with the relationship at least partially mediated by hypertriglyceridaemia3,19.
Hypothyroidism, conversely, does not appear to be associated with CAP despite frequent hyperlipidaemia. It is still not always certain whether hypertriglyceridaemia is secondary to pancreatitis or vice versa.
Complications
Pancreatitis alone produces a predictable constellation of signs relating to gastrointestinal inflammation/distress, pain of local inflammation and fluid/electrolyte disturbances. However, due to the severity of inflammation, in many cases significant complications can occur in the local vicinity of the pancreas, as well as distant/systemic organs, due to the progression to systemic inflammation.
Complications of CAP have undergone extensive research in recent years, and almost all have been associated with increased mortality risk at some stage20.
Extrahepatic biliary duct obstruction (EHBDO) is one of the more commonly recognised complications, affecting 27% of cases in one assessment21. Most define this complication by the presence of hyperbilirubinaemia and CBD dilation (more than 3mm)22. Prolonged obstruction can lead to gallbladder wall necrosis or rupture, coagulopathy and hepatocellular injury18.
EHBDO can be the most debilitating aspect for some pancreatitis patients, as evidenced by rapid resolution of clinical signs in one patient following placement of a drainage catheter23. Cholecystocentesis, potentially repeated, is a reasonable therapeutic option and may help with discomfort, though is not without risk entirely. Surgical management of EHBDO appears associated with a high mortality rate in CAP of 50%, but the process seems to resolve spontaneously given enough time in most dogs with CAP.
A survival rate of 79% has been reported in dogs with CAP/EHBDO treated medically, similar for CAP as a whole, though another study reported a lower survival rate of 57%18,22. The degree of CBD dilation (median 6.5mm) or hyperbilirubinaemia in these patients does not seem to influence the treatment choice, and evidence regarding the prognostic value of the total bilirubin concentration is mixed17,18,22.
The author agrees that most dogs with pancreatitis-associated EHBDO recover well without biliary decompression, and the degree of hyperbilirubinaemia or CBD dilation should not solely influence treatment decisions, though hepatic parameters can become scarily high (reported median peak bilirubin concentration of 182mmol/L, median peak alanine aminotransferase activity 934U/L, median peak alkaline phosphatase activity 4,288U/L)18. It can take a median of nine days to reach peak total bilirubin concentration, by which time significant reduction of clinical signs has often occurred18.
Per International Renal Interest Society classification (rapid onset of clinical signs less than one week, serum creatinine concentration increase of at least 26.5mmol/L within 48 hours), 26% to 31% of dogs with CAP developed acute kidney injury (AKI), of which 11 out of 17 were oliguric24,25.
AKI can occur with SIRS due to circulating cytokines, digestive enzymes and reactive oxygen species that can lead to endothelial damage, hypercoagulability and microthrombi deposition in the glomerulus. Coupling this with hypovolaemia and/or hypotension, and AKI is very possible. Non-survivors of CAP appear to have higher prevalence of AKI with oliguria (in fact, all dogs with normal urine output survived).
Of CAP cases that developed AKI, 71% did not survive, with an overall increased risk of 13.4 times higher for death compared to those without AKI25.
Diagnosis
Appropriate treatment and effective client communication begins with appropriate diagnosis, and this can often be the hardest part. Secondary and tertiary referrals of CAP anecdotally seem to more often be made prior to diagnosis, rather than for any specialism desired for management itself.
The reasons for this are multifactorial, but at the heart is the fact that no one test has been devised that can effectively make a definitive diagnosis.
Even histopathology, oft touted as the “true” gold standard and clearly not practical in most cases, has an unclear specificity for the clinical disease.
General recommendations
To give an idea of techniques to diagnose CAP, we can look at the inclusion criteria that studies use.
The most common criteria appear to be using cases with all three of the following: supportive clinical signs, supportive imaging findings and increased specific canine pancreatic lipase immunoreactivity (cPLI) activity (often needing more than 400 mg/L), or less commonly a positive SNAP cPLI test was allowable, too3-5,24-39. Others are happy with any two of these criteria21,40-42.
The most reasonable approach favoured by this author, as described by Fabres et al, is the collection of supportive clinical signs without an obvious alternative explanation, accompanied by either:
- Specific cPLI activity of more than 400 mg/L.
- Specific lipase activity of 200mg/L to 400mg/L or abnormal cPLI SNAP test, accompanied by supportive ultrasonographic findings43.
However, the author would also suggest that strong ultrasonographic findings can sometimes be enough, even in the absence of supportive lipase testing, if no other explanation is evident.
A full discussion of the recent evidence and opinion around the accuracy of measurement of various pancreatic lipases will not be found here. Suffice to say, the topic remains hotly debated, with some studies suggesting fantastic sensitivities and specificities; others, less so.
The weakness of its evaluation is the lack of easily definable alternative gold standard for pancreatitis to compare against.
Presentation
The average age of dogs with CAP has been reported broadly as 8 to 11 years old, reflecting that it is adult dogs, perhaps slightly older, which are most commonly affected5,20,26,34-38,41,44-46. Unsurprisingly, vomiting appears to be the most common clinical sign, generally (Table 1)28,47.
Not all affected dogs have abdominal pain, although, of course, this is likely to be under-recognised.
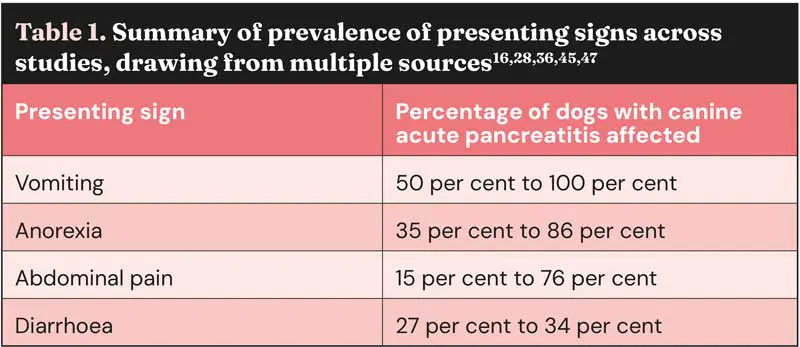
An interesting presenting sign recently described is “Cullen’s sign” in a dog with CAP and concurrent diabetes mellitus (Figure 1)48. This is an external manifestation of intra-abdominal haemorrhage that clinicians should be aware of for any underlying cause, but is especially associated with AP in people.
Ultrasonography
Abdominal ultrasonography (AUS) is historically the method of diagnosing CAP alongside lipase assessment. It is the preferred method for many, but has variable sensitivity/specificity that is strongly operator and centre dependent, influenced by how many changes need to be present to give a satisfactory diagnosis49.
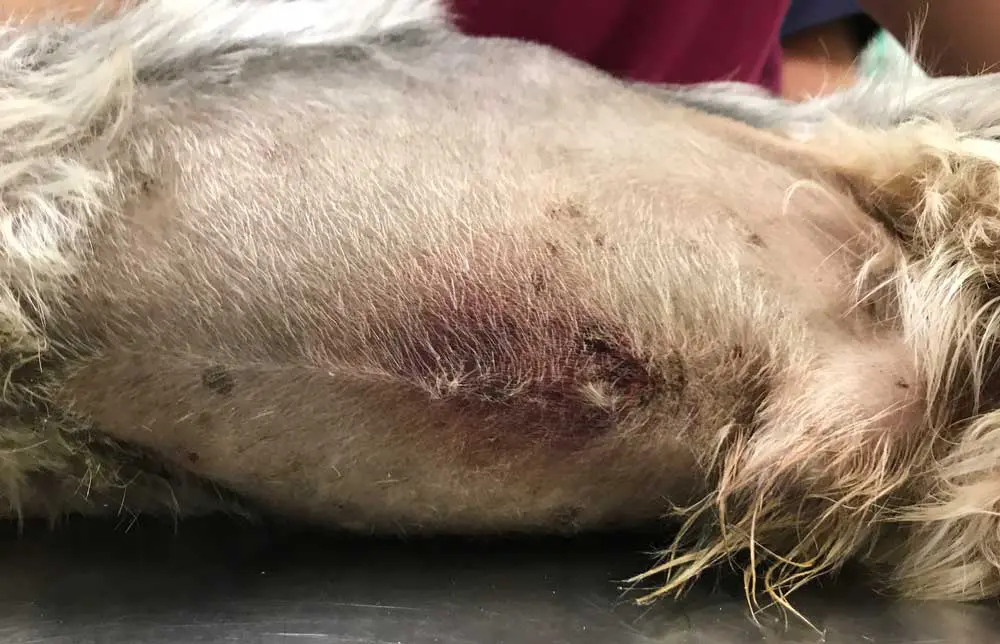
The bottom line is that AUS does not allow for the 100% confirmation or exclusion of CAP. Studies have suggested sensitivity of 70% to 88% for ultrasonographic changes, sometimes dependent on the criteria used21,40,42,43,49. Suggested criteria are identification of at least two highly suggestive changes: pancreatomegaly, pancreatic hypoechogenicity, pancreatic heterogenous echogenicity, hyperechoic mesentery (potentially the most commonly reported at 82% to 83%) and peripancreatic free fluid16,20,36,49,50. Potentially suggestive, but less important, signs are corrugated adjacent duodenum/colon, pancreatic cysts/abscesses, venous thrombosis, biliary/pancreatic duct dilation, thickening of the gastric/duodenal wall and signs of ileus36. Strong evidence exists that AUS findings change over time and may lag behind disease progression, as cPLI activity appears to increase first36,39. In two studies, at time of admission 12 out of 38 (32%) and 24 out of 37 (65%) dogs had at least two highly suggestive signs of CAP, but a further 12 out of 38 and 10 out of 37 (who mostly had no changes at all on admission) developed these after two to three days, for a total sensitivity of 68% to 92%36,39. Therefore, for dogs where CAP diagnosis is not clear but still suspected, repeating AUS after two to three days may be of significant aid (Figure 2).
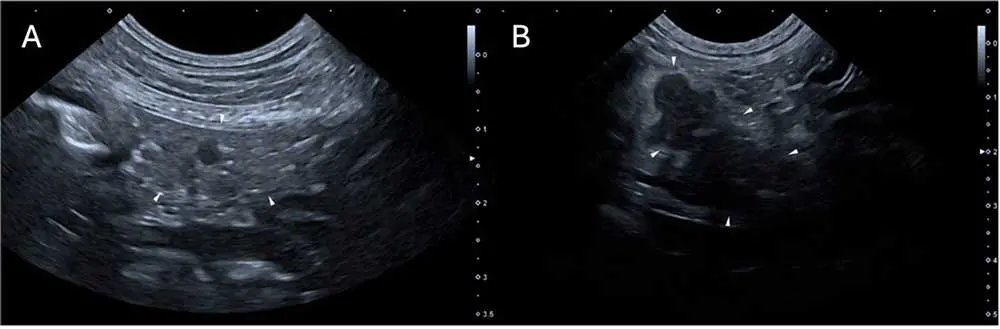
As well as pancreatic changes, CAP can also be associated with changes to the local GI tract. Forty-seven % of dogs with CAP have GI wall changes, most commonly to the duodenum (71%; Figure 3)16.
Increased heart rate was associated with increased prevalence of GI changes, suggesting it may be more often associated with severe or painful disease.
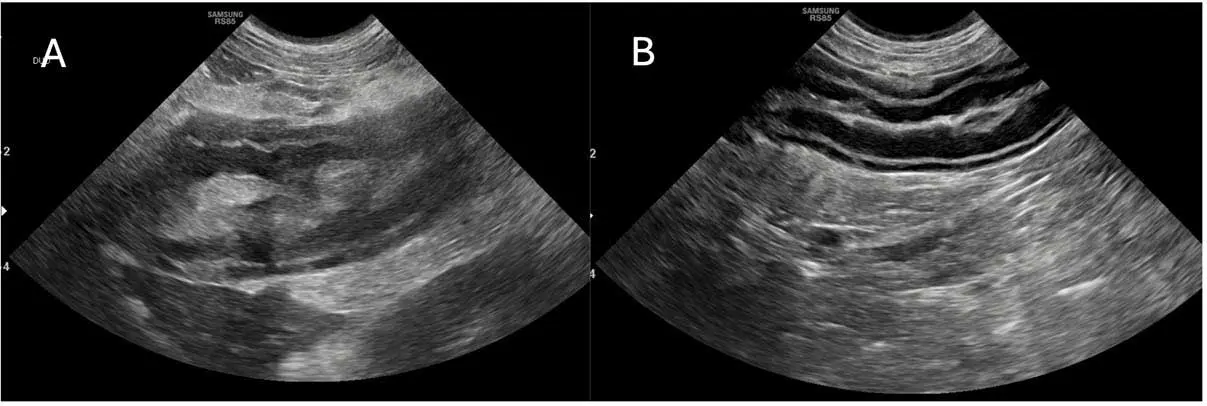
Gastric wall thickening (more than 5mm) due to presumed oedema has now been reported in association with CAP51. Most commonly, this affects the submucosal layer (but occasionally the muscularis layer, too), is usually focal (85.7% of the time) rather than diffuse, and even causes partial loss of wall layering in most cases (even complete loss, rarely).
If gastric wall thickening is seen – especially focal – then CAP should be considered as a differential (others include gastritis, usually involving the mucosal layer, too; neoplasia, usually thicker than described for CAP; ulceration; and hypoalbuminaemia).
Treatment
For most CAP patients, it is still true that no specific therapy exists. Supportive/symptomatic treatment remains the backbone (and most of the rest of the skeleton) of management. Supportive treatment typically focuses on analgesia (requirements can be very high), fluid therapy, anti-emetics, prokinetics (CAP can induce significant ileus, commonly) and nutritional intake (often mediated via feeding tubes).
Enteral nutrition is still considered one of the most important aids to recovery, with (slightly older) evidence to support this in dogs, but a strong evidence base in human medicine grounded in strong scientific theory (to preserve the mucosal barrier)52. A fuller discussion of nutritional management of pancreatitis is not presented here, but is inarguably a key topic.
The use of glucocorticoids in CAP remains an area of interest that waxes and wanes. Traditionally, glucocorticoids were considered a risk factor for development of CAP and, therefore, not particularly recommended for its treatment. However, many no longer consider this to be a risk factor, leading more to its potential therapeutic benefit as an obvious systemic anti-inflammatory medication2.
A 2019 study compared dogs with CAP treated with prednisolone (1mg/kg/day) for the duration of their hospitalisation with dogs that did not receive glucocorticoids53. Compared to the control dogs, treated dogs had lower C-reactive protein (CRP) concentrations after three days (no clear evidence that this correlates with survival), shorter time to reach lower clinical severity score, shorter hospitalisation duration (median five versus eight days) and improved survival after one month (but not survival to discharge, though a trend was noted).
Although these results offer some promise, the study was not blinded or randomised, introducing significant bias.
The overall conclusion of a recent scoping review was that fair evidence exists that glucocorticoids may reduce hospitalisation time54.
However, in most experimental studies, glucocorticoids were administered very early in the disease (even within 15 minutes), rather than the more clinically relevant situation we see in practice54. It seems to be clinician dependent as to the use of glucocorticoids in CAP at this time, but some are certainly using it23.
The author uses glucocorticoids occasionally for severe refractory cases (for example, no improvement within two to three days) – especially if EHBDO and no evidence of infection are present.
Outcomes
CAP can take a variety of paths following initial acinar injury. Some dogs will recover quickly at home, potentially without ever seeing a vet. Others can require hospitalisation for days or even weeks before recovery is seen and, as we know, it can sadly prove fatal to some – either through actual death (especially when other organs become involved) or euthanasia due to lack of perceived progress and, sadly, financial considerations in prolonged hospitalisation.
Extrapolating from our knowledge of human pancreatitis, it is likely that a significant number of dogs with CAP that are euthanised could eventually recover if given enough time in hospital, but commitment to several weeks of hospitalisation (still with an unknown eventual outcome) is reasonably not possible or desired for most owners. Average hospitalisation times have been reported as 3.5 to 5 days20,21,42,46,53. Thirty-nine % of dogs stayed longer than five days46. Mortality rates remain varied and it is difficult to compare figures, as so often the inclusion criteria and the particular component of the disease that is being looked at (for example, with or without complications) differ greatly between studies.
One-hundred % survival has been reported in one study, even with SIRS present in some4. Most report higher mortality rates of 11% to 46%17,21,24,25,29,30,32,33,39,42-44,53. The largest study of 504 dogs actually showed the lowest mortality rate of only 8.8%46.
In terms of prognostic markers for survival, many have been described in some studies, but may not be repeatable in others. When investigated, no effect was noted on mortality of age or stable pre-existing medical conditions20,30.
Reasonably good evidence exists that respiratory abnormalities are associated with mortality – dogs with higher respiratory rates, signs of respiratory distress, thoracic radiographic abnormalities or evidence of acute lung injury all were associated with death20,30,43. CRP alone does not appear able to differentiate survivors25,33.
Summary
CAP remains a disease posed to challenge us – especially diagnostically – and a disease with a worryingly high mortality rate for one we see so frequently and that is “only” inflammatory.
A combination of clinical pathology and abdominal imaging remains the optimum strategy for identifying or excluding cases, though relying on any specific one too heavily is a recipe for difficulty.
Increasing recognition of multi-systemic complications both in research and clinical practice can hopefully improve targeting of our therapies and prevention of death.
Strong, attentive, supportive and nutritional therapy remains the mainstay of treatment, often accompanied by tincture of time.
The patience sometimes required for healing is more efficiently backed up by a confident diagnosis. Despite our best efforts, CAP can be a devastating disease for some, with unavoidable death or unacceptable length of recovery.
This article has covered only some of the areas of research over the past few years relating to CAP. In the second part, the author plans to explore additional associated complications, further discussion of other diagnostic methods, and potential future/novel treatment options.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
References
- 1. Schmid SM, Hoffman JM, Gould EN et al (2024). Cross-sectional survey of 43,517 dogs in the Dog Aging Project identifies owner-reported lifetime prevalence and characteristics of gastrointestinal disease, J Am Vet Med Assoc 262(12): 1-9.
- 2. Cridge H, Lim SY, Algül H and Steiner JM (2022). New insights into the etiology, risk factors, and pathogenesis of pancreatitis in dogs: Potential impacts on clinical practice, J Vet Intern Med 36(3): 847-864.
- 3. Kim H, Kang J-H, Heo T-Y et al (2019). Evaluation of hypertriglyceridemia as a mediator between endocrine diseases and pancreatitis in dogs, J Am Anim Hosp Assoc 55(2): 92-100.
- 4. Kim H, Kim H-J, Kang J-H et al (2019). Evaluation of serum C-reactive protein and high mobility group box 1 concentrations in 22 dogs with acute pancreatitis: a pilot study, Vet Q 39(1): 122-130.
- Steiner JM, Lainesse C, Noshiro Y et al (2023). Fuzapladib in a randomized controlled multicenter masked study in dogs with presumptive acute onset pancreatitis, J Vet Intern Med 37(6): 2,084-2,092.
- Tusa NV, Abuelo A, Levy NA et al (2022). Peripheral biomarkers of oxidative stress in dogs with acute pancreatitis, J Vet Intern Med 36(6): 1,958-1,965.
- Watson P (2015). Pancreatitis in dogs and cats: definitions and pathophysiology, J Small Anim Pract 56(1): 3-12.
- Piegols HJ, Hayes GM, Lin S et al (2021). Association between biliary tree manipulation and outcome in dogs undergoing cholecystectomy for gallbladder mucocele: A multi-institutional retrospective study, Vet Surg 50(4): 767-774.
- Hattersley R, Downing F, Gibson S et al (2020). Impact of intra-operative hypotension on mortality rates and post-operative complications in dogs undergoing cholecystectomy, J Small Anim Pract 61(10): 624-629.
- Dickerson V, Poses B, Hyndman P et al (2023). Outcome in 38 dogs surgically treated for hepatic abscessation, Vet Surg 52(1): 127-133.
- Traverson M, Zheng J, Tremolada G et al (2023). Adrenal tumors treated by adrenalectomy following spontaneous rupture carry an overall favorable prognosis: retrospective evaluation of outcomes in 59 dogs and 3 cats (2000–2021), J Am Vet Med Assoc 261(12): 1-9.
- Viñeta C, Castro J, López MC et al (2024). Is pancreatitis associated with meglumine antimoniate treatment for canine leishmaniosis? A multicentric prospective study, Parasit Vectors 17(1): 1-18.
- Digiaro S, Recchia A, Colella A et al (2024). Treatment of canine leishmaniasis with meglumine antimoniate: a clinical study of tolerability and efficacy, Animals 14(15): 2,244.
- Groover J, Schaer M and Londoño L (2020). Suspected acute pancreatitis in a dog following honeybee envenomation, Can Vet J 61(4): 411-414.
- Luce BD and Hans EC (2022). Gastrointestinal foreign body obstruction is not associated with abnormal point-of-care pancreas-specific lipase test results in dogs, J Am Vet Med Assoc 260(10): 1,197-1,193.
- Hardwick JJ, Reeve JA, Reeve EJ and Hezzell MJ (2022). Prevalence of ultrasonographic gastrointestinal wall changes in dogs with acute pancreatitis : A retrospective study (2012-2020), J Vet Intern Med 36(3): 947-956.
- Guglielmini C, Crisi PE, Tardo AM et al (2022). Prognostic role of red cell distribution width and other routine clinico-pathological parameters in dogs with acute pancreatitis, Animals 12(24): 3,483.
- Wilkinson AR, DeMonaco SM, Panciera DL et al (2020). Bile duct obstruction associated with pancreatitis in 46 dogs, J Vet Intern Med 34(5): 1,794-1,800.
- Kim J, Chae Y, Lee D et al (2023). Association between hyperglycemia and canine serum pancreatic lipase immunoreactivity concentration in diabetic dogs, J Am Anim Hosp Assoc 59(5): 241-248.
- Kuzi S, Mazor R, Segev G et al (2020). Prognostic markers and assessment of a previously published clinical severity index in 109 hospitalised dogs with acute presentation of pancreatitis, Vet Rec 187(2): e13.
- French JM, Twedt DC, Rao S and Marolf AJ (2019). Computed tomographic angiography and ultrasonography in the diagnosis and evaluation of acute pancreatitis in dogs, J Vet Intern Med 33(1): 79-88.
- Cleary K, Li Chong W and Angles JM (2023). Features, management, and long-term outcome in dogs with pancreatitis and bile duct obstruction treated medically and surgically: 41 dogs (2015–2021), J Am Vet Med Assoc 261(11): 1,694-1,701.
- Chmelovski RA, Granick JL, Ober CP et al (2020). Percutaneous transhepatic cholecystostomy drainage in a dog with extrahepatic biliary obstruction secondary to pancreatitis, J Am Vet Med Assoc 257(5): 531-536.
- Gori E, Pierini A, Lippi I et al (2020). Evaluation of symmetric dimethylarginine (SDMA) in dogs with acute pancreatitis, Vet Sci 7(2): 72
- Gori E, Lippi I, Guidi G et al (2019). Acute pancreatitis and acute kidney injury in dogs, Vet J 245: 77-81.
- Mitchell L, Wang S, Lawver J and Cridge H (2024). Serial monitoring of pancreatic lipase immunoreactivity, C-reactive protein, abdominal ultrasonography, and clinical severity in dogs with suspected pancreatitis, J Vet Intern Med 38(2): 987-994.
- Yoon J-S, Kim S, Kang J-H et al (2020) Alterations in serum protein electrophoresis profiles during the acute phase response in dogs with acute pancreatitis, Can J Vet Res 84(1): 74-78.
- Berman CF, Lobetti RG and Lindquist E (2020). A comparison of ultrasonographic and clinical findings in 293 dogs with acute pancreatitis: Different clinical presentation with left limb, right limb, or diffuse involvement of the pancreas, J S Afr Vet Assoc 91(0): e1-e10.
- Gori E, Pierini A, Lippi I et al (2020). Evaluation of C-reactive protein/albumin ratio and its relationship with survival in dogs with acute pancreatitis, N Z Vet J 68(6): 345-348.
- Gori E, Pierini A, Ceccherini G et al (2020). Pulmonary complications in dogs with acute presentation of pancreatitis, BMC Vet Res 16(1): 209.
- Gori E, Pierini A, Lippi I et al (2020). Evaluation of asymmetric dimethylarginine as an inflammatory and prognostic marker in dogs with acute pancreatitis, J Vet Intern Med 34(3): 1,144-1,149.
- Lee J-H, Song W-J, An J-H et al (2021). Role of serum high-motility group box-1 (HMGB1) concentration as a prognostic factor in canine acute pancreatitis: A pilot study, Res Vet Sci 141: 26-32.
- Kuzi S, Mazaki-Tovi M, Suchodolski JS et al (2020). Protease inhibitors, inflammatory markers, and their association with outcome in dogs with naturally occurring acute pancreatitis, J Vet Intern Med 34(5): 1,801-1,812.
- Kim K, Kim H-H, Joo J-B et al (2024). Evaluation of the clinical usefulness of pancreatic alpha amylase as a novel biomarker in dogs with acute pancreatitis: a pilot study, Vet Q 44(1): 1-7.
- Neumann S (2021). Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in dogs and cats with acute pancreatitis, Vet Clin Pathol 50(1): 45-51.
- Leoni FP, Pelligra T, Citi S et al (2020). Ultrasonographic monitoring in 38 dogs with clinically suspected acute pancreatitis, Vet Sci 7(4): 180.
- Han S-M (2024). Predictive role of lactate in dogs with acute pancreatitis advanced to systemic inflammatory response syndrome, Vet Res Forum 15(3): 119-123.
- Cridge H, Langlois DK, Steiner JM and Sanders RA (2023). Cardiovascular abnormalities in dogs with acute pancreatitis, J Vet Intern Med 37(1): 28-36.
- Gori E, Pierini A, Lippi I et al (2021). Evaluation of diagnostic and prognostic usefulness of abdominal ultrasonography in dogs with clinical signs of acute pancreatitis, J Am Vet Med Assoc 259(6): 631-636.
- Von Stade L, Rao S and Marolf AJ (2023). Computed tomographic evaluation of pancreatic perfusion in 10 dogs with acute pancreatitis, Vet Radiol Ultrasound 64(5): 823-833.
- Da Silva AJ, Hope A and Mooney CT (2024). Association between hyperlipidaemia and selected cholestatic markers in 74 dogs with suspect acute pancreatitis, Animals 14(22): 3,281.
- Nielsen L, Holm J, Rozanski E et al (2019). Multicenter investigation of hemostatic dysfunction in 15 dogs with acute pancreatitis, J Vet Emerg Crit Care 29(3): 264-268.
- Fabrès V, Dossin O, Reif C et al (2019). Development and validation of a novel clinical scoring system for short-term prediction of death in dogs with acute pancreatitis, J Vet Intern Med 33(2): 499-507.
- Johnson MM, Gicking JC and Keys DA (2023). Evaluation of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and other hematologic parameters in canine acute pancreatitis, J Vet Emerg Crit Care 33(5): 587-597.
- Jandel AN, Heilmann RM, Sander H et al (2023). Serum α1-proteinase inhibitor, calprotectin, and s100a12 concentrations in the characterization of pancreatitis in dogs, Vet Sci 10(7): 428.
- Cridge H, Cabrera D, Bolton T et al (2025). Multi-institutional retrospective analysis of prognostic scoring systems for dogs with acute pancreatitis (504 dogs), J Vet Intern Med 39(3): e70114.
- Aupperle-Lellbach H, Törner K, Staudacher M et al (2020). Histopathological findings and canine pancreatic lipase immunoreactivity in normal dogs and dogs with inflammatory and neoplastic diseases of the pancreas, J Vet Intern Med 34(3): 1,127-1,134.
- Girol-Piner AM, Zamora-Perarnau C and Herrería-Bustillo V (2021). “Cullen’s” sign in a dog with acute pancreatitis, J Small Anim Pract 62(12): 1,132.
- Cridge H, Sullivant AM, Wills RW and Lee AM (2020). Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis, J Vet Intern Med 34(2): 636-643.
- Hammes K and Kook PH (2022). Effects of medical history and clinical factors on serum lipase activity and ultrasonographic evidence of pancreatitis: Analysis of 234 dogs, J Vet Intern Med 36(3): 935-946.
- Murakami M, Heng HG, Lim CK et al (2019). Ultrasonographic features of presumed gastric wall edema in 14 dogs with pancreatitis, J Vet Intern Med 33(3): 1,260-1,265.
- Cridge H, Parker VJ and Kathrani A (2024). Nutritional management of pancreatitis and concurrent disease in dogs and cats, J Am Vet Med Assoc 262(6): 834-840.
- Okanishi H, Nagata T, Nakane S and Watari T (2019). Comparison of initial treatment with and without corticosteroids for suspected acute pancreatitis in dogs, J Small Anim Pract 60(5): 298-304.
- Bjørnkjær-Nielsen K-A and Bjørnvad CR (2021). Corticosteroid treatment for acute/acute-on-chronic experimental and naturally occurring pancreatitis in several species: a scoping review to inform possible use in dogs, Acta Vet Scand 63(1): 28.
