8 Mar 2023
Advances in companion animal cancer: interventional oncology
Daniela Murgia considers the benefits and processes of pursuing this predominantly human approach in a veterinary setting.

Interventional oncology (IO) is a sub-speciality of interventional radiology, and deals with the diagnosis and treatment of cancer using targeted minimally invasive procedures performed under image guidance.
The aim of this article is to review the indications, case selection and outcomes associated with some of the IO procedures.
Cancer is a disease that benefits from a multidisciplinary approach to treatment.
Surgical resection of tumours generally offers the best long-term solution and, with certain tumour types, may result in a cure.
However, in some cases, surgery is not possible due to the size, biological behaviour, number or location of the tumour. Some patients may also be too weak to sustain open surgery, and interventional radiology (IR) treatments can be applied to provide effective and milder forms of treatment.
Interventional oncology (IO) may be applied to shrink a tumour, making a surgical or interventional treatment possible.
Although the evidence supporting the use of IO is scant in veterinary literature, much has been done in human medicine and, for many non-resectable tumours, these techniques are the first-line therapy that is pursued. IO has long been used in humans to provide palliative care for patients, reducing cancer-related pain and improve patients’ quality of life. The minimally invasive nature of the treatments causes fewer side effects and shorter recovery times, reducing costs.
Similarly, most of the current IO applications in veterinary medicine aim towards palliative treatment. IO has been established in veterinary medicine as a fourth major cancer treatment category, along with surgery, chemotherapy and radiation therapy.
IO can also be used in combination with other treatments to increase their efficacy. For example, IO techniques can be used to shrink large tumours, making them easier to excise. Chemotherapeutic drugs can also be administered intra-arterially, increasing their potency and removing the systemic side effects.
IO is mainly used:
- to restore patency to malignant obstructions through endoluminal stenting
- to provide dose escalations to tumours without increasing systemic chemotherapy toxicities via superselective trans-arterial chemotherapy delivery
- to stop haemorrhage, or reduce blood flow to tumours via trans-arterial embolisation or chemo-embolisation
- to provide therapies for those cancers with no safe or effective alternative options (Culp, 2016)
In humans, the first therapeutic embolisation procedure was reported by Barney Brooks in 1930.
Anatomy
A thorough knowledge of the anatomy is essential for performing IO procedures.
Introduction of instrumentation into, and navigation through, luminal structures requires understanding of anatomic landmarks and organ interaction, so normal blood supply can be identified from tumoural blood supply, and complications, such as non-target embolisation, can be avoided.
Diagnostic imaging
The use of imaging modalities allows for IO procedures to be performed in a minimally invasive fashion. Fluoroscopy is utilised during the majority of IO procedures.
The instrumentation – such as catheters, stents and so forth – used during IO procedures is radiopaque, and contrast agents are often injected intraluminally or intravascularly, making fluoroscopy an excellent modality for performing real-time interventions (Figure 1).
Ultrasonography is regularly used as a means of cancer staging – specifically in the abdomen; furthermore, ultrasound guidance can provide crucial assistance in obtaining tissue samples via fine-needle aspiration or biopsy. Similarly, CT and MRI are essential components of patient staging and pre-procedural planning in veterinary medicine.
Patient selection
Proper patient selection is a key element with IO procedures. When possible and appropriate, IO techniques should be considered in addition to more traditional approaches, such as surgery, chemotherapy and radiation therapy. In many scenarios in which IO options are offered, these techniques are the only available options (Culp, 2016).
IO procedures
When surgical options for tumour removal are either not feasible or not elected, loco-regional therapies can be considered as possible primary treatment options.
IO loco-regional techniques that are emerging are stenting of malignant obstructions, and intra-arterial embolisation or chemo-embolisation.
Stenting
Stenting malignant obstructions aims to restore patency through the placement of endoluminal stents.
One of the most common examples is malignant urethral obstructions associated with transitional cell carcinomas (TCCs) or prostatic tumours.
When catheterisation is not possible, alternative urinary drainage techniques are necessary. Intermittent cystocentesis can be used for short-term bladder decompression.
Concern also exists for peritoneal, body wall, and SC tracking and seeding of neoplasia – particularly TCC – if decompression via cystocentesis is used in patients with neoplasia of the urinary tract.
Cystostomy tubes have been used for urinary bypass, and are generally combined with cystopexy to reduce the risk of urine leak and uroperitoneum on removal.
The different types of cystostomy tubes include Foley urinary catheters, low profile gastrostomy tubes and mushroom tipped catheters (Figure 2).
Complications associated with cystostomy tubes include inflammation of the stoma, tube dislodgement from the bladder, damage to the tube from the patient chewing it, fistula formation following tube removal, tube obstruction, bandage sores and urinary tract infections.
Ureteral/urethral stenting is a method to provide permanent relief of obstructions secondary to neoplasia, stricture, or compression secondary to organomegaly, such as metastatic lymph node enlargement (Figure 3).
Given the generally poor response to chemotherapy seen in prostatic and transitional cell carcinomas, urethral stenting has provided an alternative to permanent cystostomy tubes in patients with obstructive urethral neoplasia. Self-expanding metallic stents, most constructed of a nickel titanium alloy known as nitinol, are generally used for urethral stenting. Urethral stents are placed under general anaesthesia using fluoroscopy (Figures 4 to 7; Clarke).
The most significant complication associated with urethral stenting is incontinence, with approximately 25% to 30% of patients being affected by severe incontinence. Due to the risk of incontinence with a permanent implant, the procedure is only performed if clients are willing to accept this possible complication. Other complications associated with urethral stenting include stent migration (rare) and stranguria. Urethral stents have also been used in cats with urethral neoplasia and urethral strictures.
Self-expanding metallic stent sizes are available in sizes optimised for feline patients, and can be placed via laparoscopic cystotomy in male cats and trans-urethrally in female cats (Culp, 2018).
Intra-arterial chemotherapy (IAC), embolisation or chemo-embolisation generally focus on local control of tumours, inducing tumour necrosis, or palliating clinical signs by direct access to the arterial blood supply, and local delivery of chemotherapy or embolic agents to the tumoural blood supply.
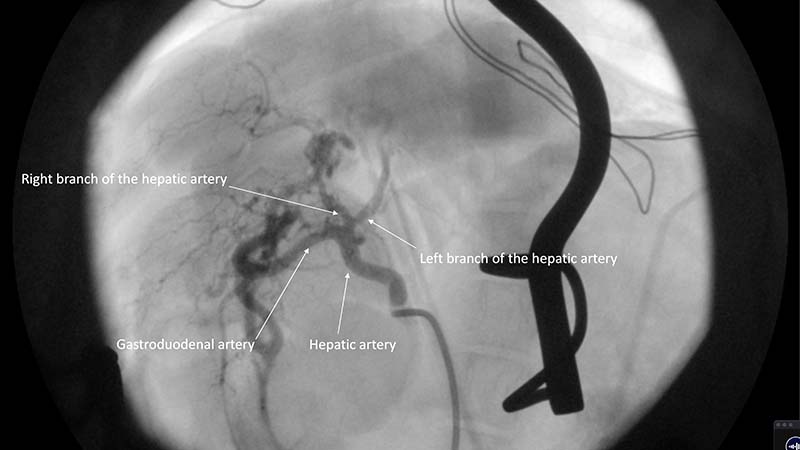
In general, the intra-arterial approach is performed through either the carotid or the femoral artery in companion animals. Selective catheterisation into the arterial blood supply of a given tumour is achieved with angiography and fluoroscopy. Depending on the nature of the mass, collateral blood supply and other structures being supplied by the artery in question, chemotherapy with or without embolic particles is delivered under fluoroscopic guidance (Figure 8).
IAC has been proposed as an advantageous use of chemotherapy compared with IV delivery because of the ability of administering higher doses of chemotherapy directly to a tumour, and decreasing potential systemic side effects associated with chemotherapy. Although a common practice in human patients, IAC administration has been rarely used in veterinary patients, but can be considered in cases where surgical resection cannot, or will not, be pursued.
Embolisation is the delivery to a certain organ and selected blood vessel of an embolic agent to cause slowing or cessation of blood flow. Embolisation is theorised to work by causing necrosis of tumour cells secondary to alteration of blood flow. When embolisation is combined with chemotherapy, the procedure is called chemo-embolisation.
A systemic dose of chemotherapy is given locally to increase drug levels within the tumour. Embolic particles are added to achieve stasis of blood flow to the tumour, which is likely more effective at controlling further tumour growth than chemotherapy.
In patients with non-resectable hepatocellular carcinomas, intra-arterial delivery of chemotherapy with or without embolic particles is an emerging interventional option for tumour management. Intra-arterial embolisation and chemo-embolisation (TAE; TACE) have been described in people, dogs and cats, with both malignant and benign hepatic neoplasia.
Most blood supply to hepatic masses is derived from the hepatic artery. In contrast, normal liver parenchyma only receives 15% to 20% from the hepatic artery. The goal of IA embolisation is occlusion of small arterioles. (Weisse, 2009; Gibson et al, 2022).
Interventional management of hepatic neoplasia
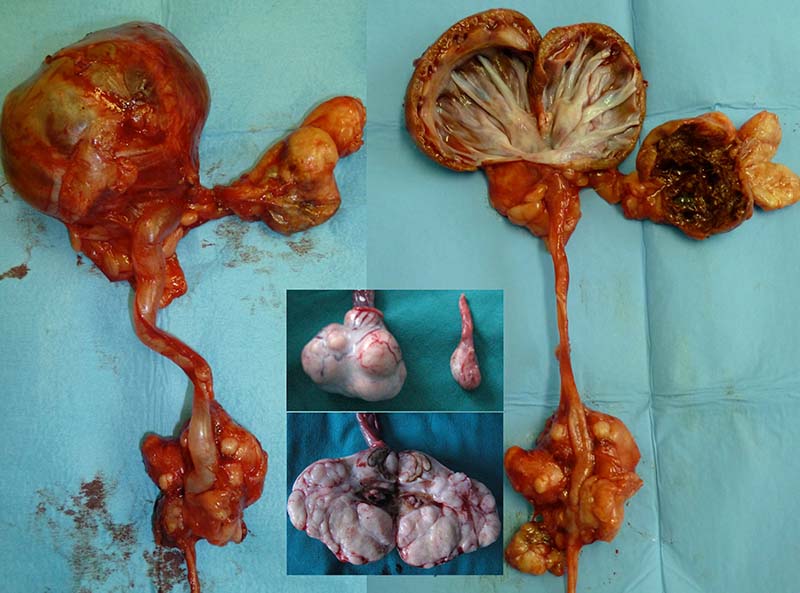
Whenever possible, resection of liver masses should be pursued because the outcomes with surgery are excellent. Many liver masses are considered “massive”, and resection of one or multiple liver lobes allows for successful control of disease. However, a subset of liver masses occurs in challenging locations (for example, surrounding major vasculature), and surgical removal may represent significant challenges or be associated with increased morbidity. In these situations, embolisation or chemo-embolisation can be considered if an adequate arterial blood supply to the mass is present.
Drug-eluting beads (DEB) are a novel method of TACE, providing a delivery system (beads) of chemotherapy in a sustained release form. Potential benefits of DEB-TACE include lower systemic levels of chemotherapy in patients undergoing DEB-TACE sparing systemic exposure compared to conventional TACE, which may enhance patient safety. In humans’ intra-arterial embolisation, fewer than 15% of masses decrease substantially in size. Instead, the mass centre becomes necrotic, but does not change substantially in size, which is commonly the case in veterinary patients, as well.
Experience with intra-arterial chemotherapy and chemo-embolisation is still in its infancy in veterinary medicine; although, preliminary experiences are promising (Culp, 2018).
Complications associated with embolisation and chemo-embolisation are uncommon; however, if an embolic agent travels to an undesired location, the effects that occur are dependent on the organ that has been embolised. Non-target embolisation of the vasculature of other organs can have serious consequences, such as pancreatitis, gastric ulceration and gall bladder infarction.
Complications of TAE, TACE, and DEB-TACE in people include haemorrhage at the vascular site, non-target embolisation, hepatic abscess formation/infarction, renal failure and post-embolisation syndrome (PES). PES is one of the most common complications of arterial embolisation and chemo-embolisation. The condition is well-described in humans, but is still being characterised in veterinary patients. It comprises pain, fever, nausea and vomiting. PES usually occurs within the first 72 hours after solid organ embolisation, such as that of liver lesions.
PES may be caused by tissue infarction and necrosis, leading to the release of breakdown products, inflammatory mediators and vasoactive substances from the embolised tissue. Symptoms are usually self-limiting and subside over time (Culp, 2018).
Fluid drainage
Interventional techniques can be used to drain both normal and malignant fluid accumulations. Drainage catheters can be placed simply with minimal sedation using a minimally invasive percutaneous technique. These catheters are generally not effective long term, but can allow for temporary stabilisation of a patient until a more permanent option is elected.
Pleural ports with IO techniques and connected to a SC port to allow for lifelong drainage.
Drainage catheters can be placed simply with minimal sedation using a minimally invasive percutaneous technique. These catheters are generally not effective long term, but can allow for temporary stabilisation of a patient until a more permanent option is elected.
Pleural ports are placed with IO techniques and are connected to a SC port allow lifelong drainage.
Pleural port
The pleural port is a subcutaneously placed delivery system, designed for patients requiring repeated access to the pleural cavity for repeated removal of pleural effusions, recurrent pneumothorax or for the delivery of medications.
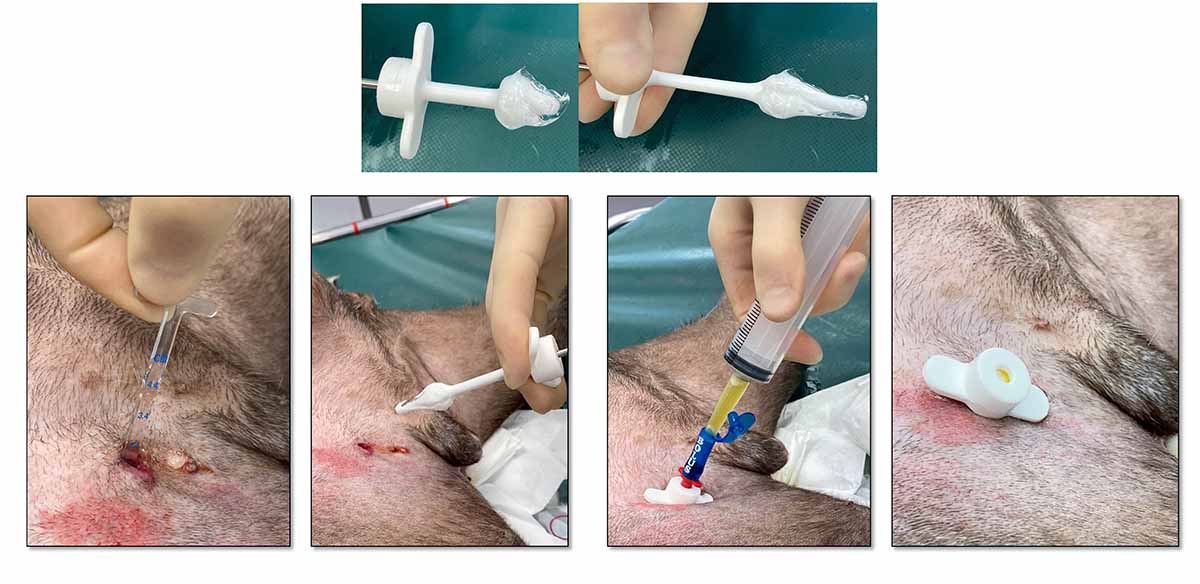
A pleural port kit includes a round tip fenestrated silicone catheter, which usually is placed within the thoracic cavity and a titanium port provided with a silicone septum, which is placed subcutaneously. This is connected to the fenestrated silicone catheter by means of a boot (Figure 9).
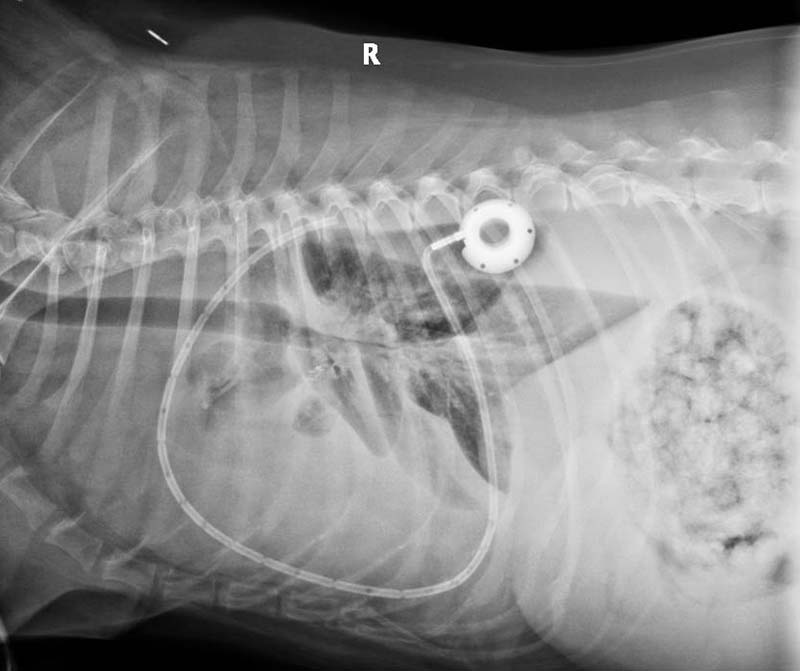
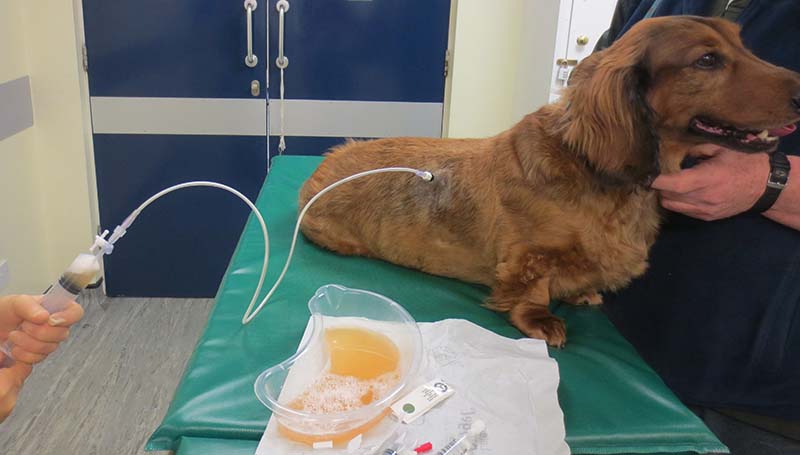
The pleural port allows a more efficient and comfortable for the patient drainage when compared to the conventional thoracostomy tubes. It eliminates inflammation and signs of pain usually associated with repeated thoracocentesis.
Due to the subcutaneous location, the risk of infection is minimised and the risk of pulmonary damage, associated with repeated thoracocentesis, is eliminated.
The pleural port is generally well tolerated and easily located by touch under the skin (Figure 10). It requires minimal maintenance and does not degrade over time.
Conclusion
IO is a growing field in veterinary medicine. Although surgery, chemotherapy and radiation therapy have their place in the treatment of cancer in companion animals, IO has the potential to supplement or improve potential outcomes.
