14 Oct 2025
Analysis and interpretation of acid-base status: part one
Nigel Dougherty BA (Zoology), BVSc, MVSc (Wildlife Health), MANZCVS (Zoo Animal Medicine), NZCert (Emergency Care) explains, in the first of this two-part series, the aim to extend practising veterinarians’ knowledge in the theory behind acid-base physiology.

Image: Thi / Adobe Stock
Metabolic processes in the body continually produce a large amount of acid and, to a lesser degree, base. Because of the reactivity of hydronium ions and their ability to alter protein conformation, they must be regulated to within tight nanomolar confines for effective homeostasis.
A potential threat to life exists if pH is less than 7.1 or more than 7.6. The body’s acid-base status, therefore, provides a critical gauge of how well homeostasis is being maintained at the cellular level and its assessment also has important clinical applications, including:
- Triage: providing important contributory information about how ill a patient is (Story, 2016).
- Diagnostics: helping narrow differentials for a patient’s condition and potentially aiding in the diagnosis of significant concurrent disease processes – particularly if performed in serial fashion (Kellum, 2000).
- Monitoring treatment: complementing lactate measurement to provide a useful clinical gauge of the appropriateness of (and response to) fluid therapy (Gillespie et al, 2017; Kilic, 2020).
Significant simple or mixed acid-base disturbances may be present in the critically ill. When pronounced (and particularly when occurring quickly), or when chronic, acid-base disturbances exert their own clinical effects (Al-Jaghbeer and Kellum, 2015; Togawa et al, 2013). The acute effects are principally cardiac and vascular in nature – particularly, reduced contractility and reduced response to catecholamines, leading to altered brain perfusion, which can be profound (De Caro Carella and de Morais, 2017). They may also alter hormone function (epinephrine, obviously, but also especially insulin) and potentially alter response to/toxicity of drug therapies through ion trapping.
Readers are referred to the paper by Al-Jagbheer and Kellum (2015) for a catalogue of the effects of acid base changes in humans, which are likely shared by veterinary patients. Critically ill patients should be regularly examined for acid-base disturbances. For those presenting with multi-organ or with cardiac, respiratory, neurological, renal, gastrointestinal or endocrine diseases, acid-base assessment may contribute important additional information to the clinical work-up. Certain facets of acid-base equilibrium are closely allied to fluid homeostasis (especially sodium homeostasis, as this relates to water retention) and to electrolyte balance (particularly sodium, potassium, chloride and calcium). As such, disturbances in one of these facets of homeostasis may affect another.
A variety of processes can alter the body’s pH, and systematic evaluation of acid-base status may allow some of these processes to be discerned from one another (Lawton et al, 2019). It is recommended that each primary acid-base disorder be recognised and assessment made to determine its possible cause, clinical significance and need for (and approach to) treatment.
Terminology
The terms alkalaemia and acidaemia, respectively, merely indicate an increased or decreased pH of blood, without indicating cause or underlying process. The terms alkalosis and acidosis describe the processes that lead to changes in production, retention or excretion of acids and bases.
It is the underlying processes that are of clinical importance, and three different approaches to the interpretation of acid base balance have been developed, to help yield more information about these processes.
The isohydric principle
Only one pH can exist (the isohydric principle), and all the various processes influencing pH may or may not collectively result in a change in pH. Therefore, the terms acidaemia and acidosis, for example, are not interchangeable; it is possible, for example, to have an overall acidaemia, but one (or more) of the processes contributing to pH could be an alkalosis – or alkaloses (De Caro Carella ana de Morais 2017).
This is because more than one process may be occurring simultaneously (in additive or opposite directions, in terms of influence on pH) or one change may occur as a physiological attempt to compensate for another.
Importantly, compensation as a process can limit changes in pH, but compensatory processes will not be enough to return pH to normal. Because compensation is never fully effective, it is usually possible to identify the primary underlying process by reference to the direction of pH alteration and by accounting for expected extents of physiological compensation.
Importantly, normal pH may not necessarily imply sound acid-base homeostasis.

Normal pH values
So, what are the normals that allow us to define acidaemia and alkalaemia? Using venous blood gas measures for dogs, a useful number to remember is the number four as normal, with anything deviating more than 0.4 from this as abnormal.
Dogs
- Normal pH is 7.4.
- Increased pH of more than 7.44 is alkalaemia.
- Decreased pH of less than 7.36 is acidaemia.
Cats
Cats, physiologically, have more acidic blood than dogs. For them, remember the number three as normal, with anything deviating more than 0.4 from this as abnormal.
- Normal pH is 7.3.
- Increased pH of more than 7.34 is alkalaemia.
- Decreased pH of 7.26 is acidaemia.
What are the principal determinants of pH?
Once we know the pH, we need to ask: what are its principal determinants?
Buffers
The most important initial determinant of pH are the body’s buffers (De Caro Carella and de Morais 2017). A buffer consists of a mixture of a weak conjugate acid-base pair (carbonic acid as the conjugate acid and carbonate as the conjugate base is a good example, and this acts as a buffer, even without expiratory control of carbon dioxide levels) with suitable abundance and dissociation constants that make the pair capable of reacting with or generating hydronium ions in a manner finely dependent on ambient pH.
For a buffer to work, both the acid and the base component must be part of the same thermodynamic equilibrium system – that way, reactions involving one or other component (by adding strong acid or base) will transform it into its conjugate base pair, so perpetuating the buffer mixture.
The body’s buffer response, which is immediate in its initiation, varies in make up between the intracellular and interstitial compartments. Proteins are the most important intracellular buffers. Proteins contain positively charged amino groups and negatively charged carboxyl groups (which, respectively, bind hydrogen and hydroxyl ions and, therefore, function as buffers).
The buffer systems functioning in blood plasma include the bicarbonate and carbonic acid buffers, proteins and phosphates. Buffering by proteins accounts for two-thirds of the buffering power of the blood, and haemoglobin is the most important blood buffer protein, whose buffering capacity depends on whether it is in reduced state.
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4−) – a weak acid, and sodium monohydrogen phosphate (Na2HPO42-) – a weak base. The bicarbonate buffer is the primary buffering system of the interstitial fluid surrounding the cells in tissues throughout the body. With 20 times more bicarbonate than carbonic acid in the blood, this system is most efficient at buffering changes that would make the body more acidic.
Interstitial fluid tends to receive acid loads, and the intracellular space receives cations such as potassium in exchange for acid loads. This is important, as cells work better in a slightly alkaline environment.
Respiratory and metabolic determinants
Once buffering capability reaches equilibrium (usually within 30 minutes), the remaining determinants of pH are conceptually and mechanistically differentiated into respiratory and metabolic contributory components (a somewhat simplistic differentiation due to the concept of variance, which will be touched on in this article).
The respiratory component
No matter what approach to acid base interpretation is used, the interpretation of the respiratory component is always the same.
The respiratory component governs the excretion of carbon dioxide (as increases in its production rather than problems with its excretion are rarely of clinical concern), which in turn has an influence on bicarbonate levels. Think of the respiratory component as the airway, the lungs, the pleural space, the respiratory muscles, a normal central respiratory control (the brain) and, as importantly, the blood supplying the lungs (ventilation-perfusion matching)(Johnson and de Morais, 2012).
So, what are the respiratory normals?
Dogs
For dogs, once again it is the number four that is useful to remember.
- Normal pCO2 is 40mmHg (with a normal range of 36mmHg to 44mmHg).
Cats
For cats, it is slightly less.
- Normal pCO2 is 36mmHg (with a normal range of 34mmHg to 38mmHg). An abnormal pCO2 is a deviation of greater than 4mmHg from the midrange.
For dogs, pCO2 of less than 36mmHg or more than 44 mmHg means a respiratory component to the acid/base picture exists (but not necessarily that a respiratory problem exists – think again of compensation).
A brief word about ventilation-perfusion matching, which can be helpful to assess in anaesthetised patients. Assuming satisfactorily tight endotracheal tube cuffing is attained, venous pCO2-ETCO2 gradients exceeding 15mmHg may crudely reflect reduced pulmonary blood flow as a cause of inappropriate alveolar dead space, although it should be noted that variable venous gradients in this region are often observed in larger mammals.
The metabolic component
The metabolic component essentially encompasses all the non-respiratory processes that can influence body pH. Perhaps the best definition of metabolic disturbances are those that are the consequence of disease processes leading to alterations of the total concentration of weak acids ([Atot]) or strong ion difference (SID), which will be defined in more detail below (De Caro Carella and de Morais, 2017). This, in turn, leads to alterations in pH and [HCO3–].
The development of three different approaches to the characterisation of the metabolic component can be confusing in terms of equivalence between parameters used in the different approaches to partition and scale the various metabolic components (Kellum, 2000). Since the traditional, descriptive carbon-dioxide/bicarbonate equilibrium approach was first proposed by Henderson and Hasselbalch, two other approaches have been developed to try to address the shortfalls of the traditional approach, principal among which are:
- The need to better accommodate for variance in the relationship between drivers of bicarbonate and carbon dioxide levels.
- The need to embrace the physical chemistry of what induces the dissociation of water to generate hydronium ions and, therefore, what the body must modulate.
- The need to better accommodate for the compartmental nature of acid base balance and its influences – however, models for doing this are only suitably advanced in human medicine (Anstey, 2010; Wolf and Deland, 2011; Wooten, 1999; Wooten, 2003).
The traditional approach
The metabolic component in the traditional approach essentially describes all the other, non-respiratory processes that influence the generation, conservation, consumption or loss of the biochemical buffer, bicarbonate (as it is quite rare to have other bases produced or lost). In this approach, the quantification of bicarbonate provides indication of the scale of metabolic imbalance, but it is important to note that bicarbonate is simply a measure of metabolic imbalance; it is not a primary determinant of pH.
- Dogs: normal [HCO3–] is 24mEq/L with a normal range of 20mEq/L to 28mEq/L. Some references, such as Hopper and Epstein (2012), quote a range of 20mEq/L to 24mEq/L.
- Cats: again, cats like to be different and have slightly lower normal bicarbonate than dogs. Normal [HCO3–] is 21mEq/L with a normal range of 17mEq/L to 25mEq/L. Some references, such as Hopper and Epstein (2012), quote a range of 18mEq/L to 23mEq/L.
If the [HCO3–] is abnormal, then a metabolic component to the acid-base status exists (but not necessarily a metabolic problem – think once again of compensation). In mixed disorders, interpreting [HCO3–] can be misleading. Unfortunately, [HCO3–] is obviously also influenced by carbon dioxide levels (not surprising, given carbonic acid’s reversible dissociation into carbon dioxide and water under the influence of carbonic anhydrase). In fact, in none of the approaches are the adopted metabolic measures truly invariant to carbon dioxide levels. However, quantifying bicarbonate relative to carbon dioxide helps in characterising disorders and identifying if compensatory processes may be occurring.

The semiquantitative approach
In the semiquantitative approach, the term standardised base excess (SBE) has been developed to provide a quantification of the metabolic imbalance.
To try to control for the confounding effect of carbon dioxide, SBE is a measure of the molar amount of acid or base that must be titrated to return pH to normal, after controlling for carbon dioxide levels (derived from van Slyke equations) and after accommodating for the influence of important buffers (such as haemoglobin and other proteins). From an equivalence point of view, the SBE is a measure of the change in SID from its normal equilibrium, as depicted in the quantitative approach.
- Dogs: Normal SBE: -4mmol/L to -1mmol/L.
- Cats: Normal SBE: -5mmol/L to 0mmol/L (Hopper and Epstein, 2012).
The quantitative approach
In the Stewart or quantitative approach, the association between electrolytes and pH is made most explicit, as some electrolytes act as strong Brønsted acids and bases because acid electrolytes generate hydronium ions in solution (for example, HCl) and basic electrolytes bind hydronium ions to available binding groups (for example, OH–). Furthermore, since the body’s maintenance of electroneutrality is paramount, acid base balance may need to be sacrificed if specific ions need to be retained (Hopper, 2019). The Stewart approach measures the respective contribution of those parameters, which theoretically have an independent (rather than dependent) influence on pH – in other words, it quantifies the substances that are considered to primarily influence the dissociation of water and, therefore, emphasises what the body actually must regulate in order to achieve pH homeostasis (Stewart, 1983; Wooten, 2004).
Key among these are the electrolytes most prevalent in biological systems: total carbon dioxide (as carbonic acid/bicarbonate), sodium and chloride. Other important ions may also exist that are not routinely measured and usually (but not universally) these are anions. The combined valence, concentration and propensity to yield or bind hydronium ions of all are what will primarily influence the dissociation of water to generate (or neutralise) hydronium ions.
While Stewart’s approach is embraced as mechanistic, others argue that, like Henderson and Hasselbalch’s approach, Stewart’s equations do not offer any cause-and-effect value and are equally descriptive. Irrespective, according to Stewart, the three key independent determinants of pH are:
pCO2 (just as with the traditional descriptive and semiquantiative approaches).
The SID or valent difference between strong (such as fully or near fully ionised) anions and strong cation concentrations (particularly the difference between sodium and chloride concentrations, as they are relatively the most abundant and variable by far).
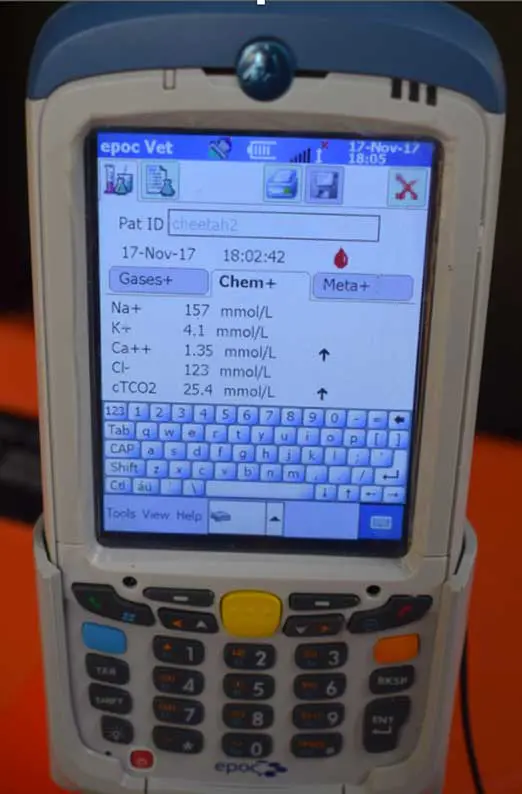
Normal values are as follows:
- Dogs: sodium 145mmol/L to 154mmol/L.
- Cats: sodium 149mmol/L to 155mmol/L.
- Dogs: chloride 112mmol/L to 118mmol/L. Hyperchloraemia is chloride more than 118mmol/L.
- Cats: chloride: 118mmol/L to 124mmol/L. Hyperchloraemia is chloride more than 124mmol/L (Hopper and Epstein, 2012).
However, beware of assigning too much significance to one or other of these ions; it is the balance between these two that is important.
- Weak acids – especially albumin and phosphate concentrations (collectively termed A–);
- Another, calculated determinant exists, the measure of which is termed SIG (Story, 2008). This measure accounts for all those “covert” ions that remain unmeasured, which permit the satisfaction of the law of electroneutrality. Relatively more anions make for more hydronium ions; more cations makes for less hydronium ions. SIG, which should equal zero, is measured as follows (Figge et al, 2018; Figge et al, 1992; Kellum et al, 1995; McCullough and Constable, 2003):
- SIG = SIDa-SIDe, where:
- SIDa = (Na+ + K+ + Ca2+) – (Cl- + Lac-)
- SIDe = 1,000 × 2.46 × 10-11 × (pCO2/10pH) + A–
- And:
- A– = [albumin] × (0.123 × pH – 0.631) + [phosphate] × (0.309 × pH – 0.469).
In the traditional approach, a similar calculated determinant is known as the anion gap, but anion gap and SIG are not mathematically equivocal because anion gap does not account for the influence of A– in its calculation (Artero, 2017). It also has a number of interpretative weaknesses in circumstances of critical illness (Al-Jaghbeer and Kellum, 2015).
Normal anion gap, including potassium, is abbreviated as AG(K) = (Na + K) – (HCO3 + Cl), and is as follows:
- Dogs: 9mmol/L to 16mmol/L. Metabolic acidosis associated with increased AG: AG more than 16mmol/L.
- Cats: 16mmol/L to 20 mmol/L. Metabolic acidosis associated with increased AG: AG more than 20mmol/L.
This article appeared in Vet Times (2025), Volume 55, Issue 41, Pages 14-18
Nigel Dougherty is a Kenya citizen who works as a wildlife and emergency and critical care veterinarian and zoologist. He is the author of Wild Vet Walkabout, an illustrated veterinary travelogue, and 50 per cent of the book’s sales profits will be donated to the veterinary work of the David Sheldrick Wildlife Trust.
References
- Al-Jaghbeer M and Kellum JA (2015). Acid–base disturbances in intensive care patients: etiology, pathophysiology and treatment, Nephrol Dial Transplant 30(7): 1,104-1,111.
- Anstey CM (2010). Estimating the net effect of unmeasured ions in human extracellular fluid using a new mathematical model. Part I: Theoretical considerations, Anaesth Intensive Care 38(5): 862-869.
- Artero CT (2017). A quick reference on anion gap and strong ion gap, Vet Clin North Am Small Anim Pract 47(2): 191-196.
- De Caro Carella C and de Morais HA (2017). Compensation for acid-base disorders, Vet Clin North Am Small Anim Pract 47(2): 313-323.
- Figge J et al (1992). Serum proteins and acid-base equilibria: a follow-up, J Lab Clin Med 120(5): 713-719.
- Figge J et al (2018). Quantitative relationships among plasma lactate, inorganic phosphorus, albumin, unmeasured anions and the anion gap in lactic acidosis, J Crit Care 44: 101-110.
- Gillespie I et al (2017). Update: clinical use of plasma lactate, Vet Clin North Am Small Anim Pract 47(2): 325-342.
- Hopper K (2019). Acid-base, electrolyte and endocrine disorders. In Drobatz KJ et al (eds). Textbook of Small Animal Emergency Medicine (1st edn), John Wiley and Sons, Hoboken: 681-689.
- Hopper K and Epstein SE (2012). Incidence, nature, and etiology of metabolic acidosis in dogs and cats, J Vet Intern Med 26(5): 1,107-1,114.
- Johnson RA and de Morais HA (2012). Respiratory acid-base disorders. In DiBartola S (ed), Fluid, Electrolyte and Acid Base Disorders in Small Animal Practice (4th edn), Elsevier Saunders, St Louis: 287-301.
- Kellum JA (2000). Determinants of blood pH in health and disease, Crit Care 4(1): 6-14.
- Kellum JA et al (1995). Strong ion gap: a methodology for exploring unexplained anions, J Crit Care 10(2): 51-55.
- Kilic O et al (2020). The impact of intravenous fluid therapy on acid-base status of critically ill adults: a Stewart approach-based perspective, Int J Nephrol Renovasc Dis 13: 219-230.
- Lawton TO et al (2019). Perioperative metabolic acidosis: The Bradford Anaesthetic Department Acidosis Study, J Intensive Care Soc 20(1): 11-17.
- McCullough SM and Constable PD (2003). Calculation of the total plasma concentration of nonvolatile weak acids and the effective dissociation constant of nonvolatile buffers in plasma for use in the strong ion approach to acid-base balance in cats, Am J Vet Res 64(8): 1,047-1,051.
- Stewart PA (1983). Modern quantitative acid-base chemistry, Can J Physiol Pharmacol 61(12): 1,444-1,461.
- Story DA (2008). Filling the (strong ion) gap, Crit Care Med 36(3): 998-999.
- Story DA (2016). Stewart acid-base: a simplified bedside approach, Anesth Analg 123(2): 511-515.
- Togawa A et al (2013). Adjusted anion gap is associated with glomerular filtration rate decline in chronic kidney disease, Nephron Extra 3(1): 113-117.
- Wolf MB and Deland EC (2011). A comprehensive, computer-model-based approach for diagnosis and treatment of complex acid-base disorders in critically-ill patients, Journal Clin Monit Comput 25(6): 353-364.
- Wooten EW (1999). Analytic calculation of physiological acid-base parameters in plasma, J Appl Physiol 86(1): 326-334.
- Wooten EW (2003). Calculation of physiological acid-base parameters in multicompartment systems with application to human blood, J Appl Physiol 95(6): 2,333-2,344.
- Wooten EW (2004). Science review: quantitative acid-base physiology using the Stewart model, Crit Care 8(6): 448-452.
