6 Oct 2020
Arthritis in feline patients – best advice to give owners
Karen Perry discusses the diagnosis and treatment of OA, and the importance of cat owner education in their success.
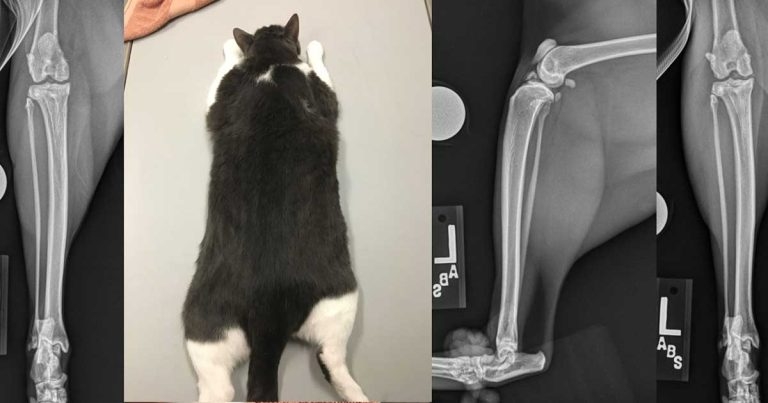
Despite a high prevalence, feline OA remains underdiagnosed and undertreated. The clinical signs associated with OA in cats are subtle and non-specific, and this certainly contributes to this incongruity. The relative rarity with which vets are able to observe normal behaviour in cats is an additional factor. As such, owners play a critical role in the diagnosis and monitoring of OA-associated pain in cats, with recognition relying heavily on owner-reported changes in behaviour.
Given that a diagnosis is a necessary prerequisite for successful treatment, perhaps one of the most substantial strides forward that we can make in feline OA management, is to better educate cat owners, and to recognise and use them as a valuable resource. Education of owners, in terms of what signs to be monitoring for, may improve our abilities to diagnose and treat appropriately at an earlier stage in the disease process.
Clinical metrology instruments and quality of life questionnaires play an increasingly important role in the identification and monitoring of chronically painful conditions. The strategic employment of such tools, in collaboration with owner education and engagement, has the potential to result in improved screening, monitoring and, hence, treatment of feline OA.
OA is common in cats, with an estimated prevalence of between 40% and 90% based on radiographic detection of joint changes (Clarke et al, 2005; Hardie et al, 2002; Lascelles et al, 2010; Slingerland et al, 2011; Lascelles et al, 2012).
In cats, the hip, stifle, hock and elbow joints are the most commonly affected synovial joints (Lascelles et al, 2010; Figure 1). Although much less is known about the aetiology of OA in cats as compared to dogs, idiopathic OA is considered common, with congenital, traumatic, infectious, nutritional and immune-mediated causes also having been documented (Lascelles et al, 2010).
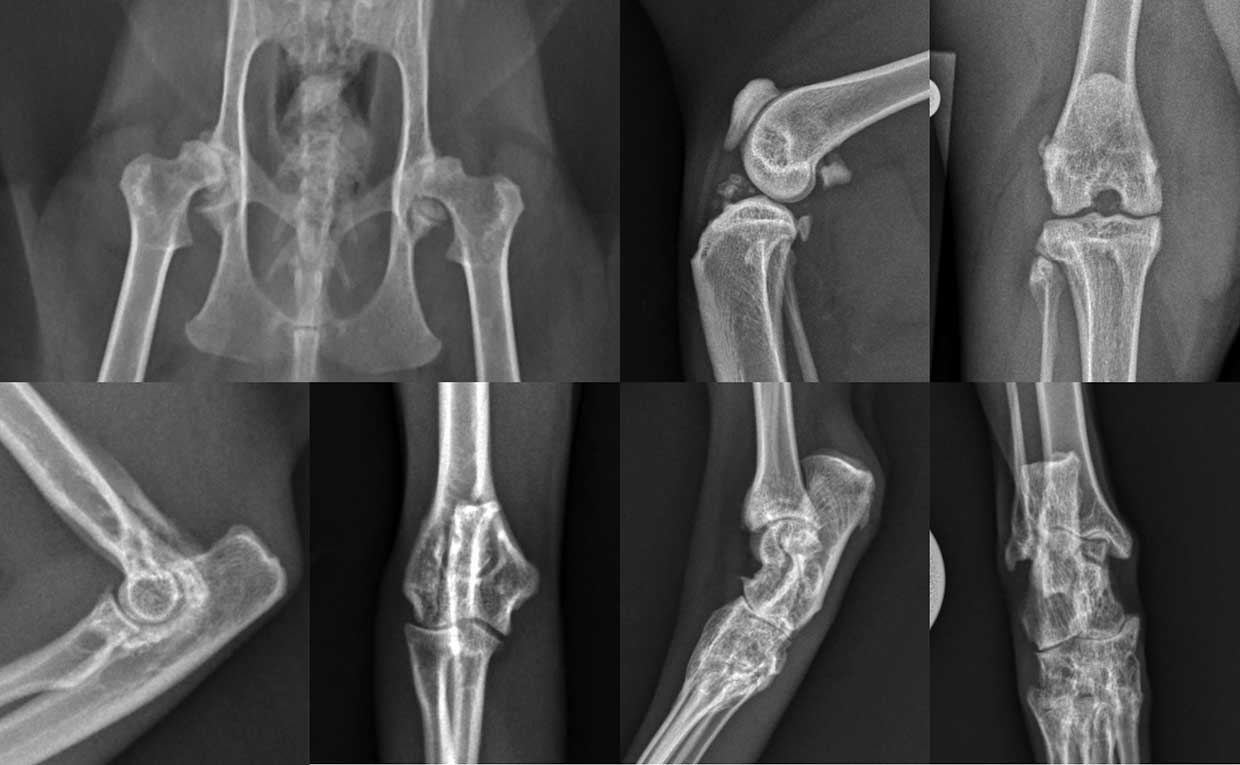
OA is characterised by progressive degradation and loss of articular cartilage, related to altered intrinsic mechanisms of the cartilage, influenced by changes in other intra-articular tissues such as the synovium and subchondral bone (Wei and Bai, 2016).
The progressive deterioration of one or more components of the joint is associated with the development of chronic pain, which in turn negatively affects quality of life, causes changes in behaviour, and impacts health and welfare, as well as the owner-companion animal bond (Mathews et al, 2014; Monteiro and Lascelles, 2017).
For most chronic painful conditions, inflammation is an important triggering factor. In OA, chronic tissue damage and low-grade inflammation produce and release inflammatory mediators, which activate nociceptors and promote a cycle of further joint destruction (Mobasheri and Batt, 2016).
The development of joint inflammation and the release of inflammatory mediators render peripheral nociceptors hyperexcitable (Enomoto et al, 2019) and, subsequently, result in their sensitisation. The sustained nociceptive input from the periphery to the spinal cord additionally contributes to development of central sensitisation (Klinck and Troncy, 2016). These mechanisms are generally short lived and reversible; nevertheless, in the face of sustained inflammation and/or nerve injury, they can become pathologic.
The fact chronic pain results from a mixture of inflammatory, neuropathic and functional components is important for owners to understand as this is the reason why OA has a multitude of clinical presentations. It also explains why responses to therapy within the same clinical condition can vary so widely, and can help cat owners comprehend the inherently challenging nature of diagnosis, treatment and monitoring of treatment response with this condition.
Diagnosis
In spite of the high prevalence of OA in cats, OA remains underdiagnosed and undertreated (Taylor and Roberston, 2004; Bennett et al, 2012). Several reasons for this discrepancy have been suggested, including cats being presented to vets less frequently and the intrinsic difficulty of diagnosis of OA in cats. Another reason may be the lower prevalence of single-limb lameness as a major clinical sign of OA in cats (Lascelles, 2010).
While owners are generally familiar with lameness as a classical sign of joint and other limb pain, in the absence of this sign, they often attribute other behavioural signs of OA to normal ageing change. This is particularly problematic with cats as they generally perform very differently in the veterinary clinic than they do at home and rarely demonstrate normal behaviours. This lowers the opportunity for veterinary observation of important behavioural signs.
Therefore, along with veterinary orthopaedic examination and radiographs, owners remain a critical part of the diagnosis and monitoring of OA-associated pain in cats (Clarke et al, 2005; Hardie et al, 2002; Suter et al, 1998; Enomoto et al, 2020).
Clinical signs of chronic pain are generally subtle and progress slowly. Recognition relies mostly on owner-reported changes in behaviour (expression of new behaviours and disappearance of old behaviours; Monteiro, 2020). Therefore, education of owners, in terms of what signs to be monitoring for, may improve our abilities to diagnose and treat appropriately at an earlier stage in the disease process.
In terms of mobility and ability to perform activities, several signs can be associated with OA in cats, including the peaks and troughs of activity becoming less extreme; the cat’s movement becoming more stiff, and less fluid and graceful; the daily distance moved by the cat decreasing; and the cat demonstrating difficulty in getting up after a long sleep or rest.
Many cats are reported to hesitate or start to avoid jumping up, or down, with decreased jumping up being associated with pelvic limb disease and a reluctance to jump down being associated with thoracic limb OA.
Owners sometimes report that cats have started to use steps to reach high or low areas rather than jumping up or down, while other owners reported that cats demonstrate difficulty in getting up or down stairs. It is fairly frequently reported that cats lose interest in play and exploratory activities, and many cats are reported to alter their posture while sitting or lying down (Figure 2).

In terms of social interactions, some cats decrease the level of interaction with their owners and with other animals in the house, often becoming more isolated, and seeming less friendly and more irritable. Adverse reactions are often noted when cats are lifted up and they may lose interest in activities that used to stimulate them, such as toys or distractions through the window.
Owners often also report alterations in self-care associated with OA, including the hair coat becoming greasy and the nails dirty. Decreased scratching and stretching behaviour may be noted – sometimes with an associated perceived overgrowth of the nails – while some cats demonstrate excessive grooming, leading to areas of alopecia.
Elimination habits are occasionally affected, including misuse of the litter box due to difficulty getting in or out, or a failure to bury faeces, which is often associated with thoracic limb OA. Changes in food and water intake may also be noted, including both the quantity ingested and the associated behaviours. In some cases, hypersensitivity reactions are also noted. This may include resentment to being touched, petted or brushed; sudden episodes of vocalisation and running away for no apparent reason; and sudden episodes of looking at a region of the body, followed by licking, hair plucking or biting.
In the veterinary clinic, these hypersensitivity reactions may manifest as an aversive response to pain during an orthopaedic examination. Cats with OA show significant differences in responses during orthopaedic examination when compared with sound cats (Enomoto et al, 2020) and are less friendly (Lascelles et al, 2012). These behaviours might be indicative of negative emotions related with long-standing pain and consequent poor animal welfare states (Monteiro, 2020).
The importance of cat-owner education regarding OA in general and typical associated clinical signs has been documented. Studies have shown wide gaps between the responses of owners who are informed about OA and those who are not, when it comes to assessing behaviour changes associated with OA (Enomoto et al, 2020). This gap represents an opportunity for engagement and education of owners with adult and senior cats. As cats are most likely to perform these behaviours at home, rather than at the clinic, owner engagement is critical to the detection and diagnosis of OA, and associated pain (Enomoto et al, 2020).
In the clinical setting, owner-reported behavioural signs remain the best assessment tool of feline chronic pain. Information about specific behaviours is collected using clinical metrology instruments (CMIs), quality of life (QoL) or health-related QoL (HRQoL) questionnaires. These instruments are constructed based on intensive research, which has identified and validated key behaviours that are indicative of pain or QoL. They generally include questions pertaining to mobility, ability and willingness to perform activities, sociability and self-care (Monteiro, 2020).
Several CMIs, QoL and HRQoL questionnaires have been published for use in cats with chronic painful conditions. These include, but are not limited to:
- the Cat Health and Wellbeing HRQoL (Freeman et al, 2016)
- the Feline Musculoskeletal Pain Index (FMPI) CMI (Gruen et al, 2014; Zamprogno et al, 2010; Benito et al, 2013a; 2013b)
- the Feline Musculoskeletal Pain Screening Checklist (Feline MiPSC; Enomoto et al, 2020)
- the Feline QoL HRQoL (Tatlock et al, 2017)
- the Montreal Instruments for Cat Arthritis Testing for use by both caretakers (Klinck et al, 2018a; 2018b), and veterinarians (Klinck et al, 2018c; Klinck et al, 2015).
The Feline MiPSC was recently developed in an effort to increase awareness of OA in cats, and to provide a quick, easy and practical tool for screening of cats for OA-associated pain (Enomoto et al, 2020). It is used as a foundation for discussion of feline OA with owners and to establish the need for further veterinary investigation.
The Feline MiPSC is comprised of six items asking if a specific activity can be performed normally:
- Does your cat jump up normally?
- Does your cat jump down normally?
- Does your cat climb up stairs or steps normally?
- Does your cat climb down stairs or steps normally?
- Does your cat run normally?
- Does your cat chase moving objects (toys, prey and so on)?
If any of the items is scored as a “no” (that is, the activity is not normal), this should prompt further evaluation with a more detailed screening, such as a review of a video taken in the home environment or in-clinic observation and orthopaedic evaluation (Enomoto et al, 2020). As such, the Feline MiPSC can be used as a screening tool, whereas other tools such as the FMPI or Montreal Instrument for Cat Arthritis Testing might be used for more in-depth investigation, or for monitoring treatment efficacy (Enomoto et al, 2020).
The Feline MiPSC is not a tool to be used in isolation, but more one to be used in conjunction with the owner education regarding clinical signs associated with OA as previously described.
The use of the Feline MiPSC was evaluated in owners informed about OA and in owners who were not informed. While the positive predictive value of the checklist was greater than 97% in both informed and non-informed owners, meaning there were few false-positive screening test results, the negative predictive value varied between groups (Enomoto et al, 2020).
The negative predictive value of the checklist was moderate (56.3%) in the non-informed group and strong (88.9%) in the informed group. This suggests that, especially when owners are not aware of the behavioural effects of OA in cats, cats with negative results on the checklist may still have OA. Again, this highlights the importance of owner education and engagement (Enomoto et al, 2020).
However, when coupled with educational tools designed to engage owners in monitoring their cats for behaviours associated with painful OA, the Feline MiPSC will serve two purposes. First, it will be able to increase vets’ ability to assess for OA in a clinically expedient manner; second, it will provide a foundation for increasing awareness of OA among cat owners (Enomoto et al, 2020). This may translate into earlier diagnosis and treatment of OA in cats, and in these cases, treatment may be more efficacious.
Treatment
Non-pharmacologic treatment
Treatment of chronic pain relies on the use of pharmacologic and non-pharmacologic approaches. While the pharmacologic treatment of feline OA is fairly commonly discussed, the importance of non-pharmacologic treatment is often underappreciated.
The potential benefits of non-drug therapies in the management of chronic pain are enormous – particularly as our understanding regarding the contribution of affective states to pain perception increases (Roy et al, 2009; Melzack and Katz, 2013).
While further work in this aspect of pain management in veterinary medicine is necessary, it is generally accepted that a positive emotional and mental state decreases pain, and vice versa. Therefore, promoting a positive emotional state can potentially provide analgesia and improve feline welfare (Monteiro 2020). Additionally, owners can be actively engaged in this component of the OA-management plan, which may facilitate maintenance of the owner-cat bond.
A sedentary lifestyle and obesity are common findings in domestic cats (Figure 3), and both factors are known to contribute to chronic pain and should be managed as a component of non-pharmacologic OA treatment.
Physical rehabilitation and weight control play an important part in preventing and managing chronic pain (Monteiro, 2020), and represent an important component of a tailored OA-therapeutic strategy. Environmental enrichment to promote the expression of species-specific behaviours provides physical and mental stimulation, and this modality may decrease pain and stress, while increasing activity and mobility.
Many resources are available that may help owners develop an appropriate environment for their pet – particularly for indoor-housed cats (Ellis et al, 2013; Dantas et al, 2016; Heath and Wilson, 2014).
Psychological treatments, including cognitive behavioural therapy are used in human medicine for the treatment of chronic pain, with strong evidence for reducing pain, disability and anxiety (Eccleston et al, 2014; Kroon Van Diest and Powers, 2019). Similarly, cognitive enrichment of shelter cats has been shown to improve measures of contentment and health (Gourkow and Phillips, 2016).
Although data is lacking on the effects of cognitive enrichment on chronic pain in cats, strategies to provide mental stimulation, such as environmental enrichment and play sessions, are strongly recommended and represent one way to maintain the owner-companion animal bond (Monteiro, 2020).
Massage reduces stress, pain, tension and discomfort in paediatric chronic pain patients (Suresh et al, 2008) and, while evidence is lacking in cats to date, owners can easily be trained to perform massage and passive range of motion exercises (Monteiro, 2020). Transcutaneous electrical nerve stimulation, photobiomodulation therapy and targeted pulse electromagnetic field therapy may have the potential to reduce pain and inflammation, but no data is available in cats to date (Monteiro, 2020).
Pharmacologic treatment
In the early stages of feline OA, non-pharmacologic therapy alone may be effective. However, as the condition progresses and pain increases, analgesics may be added to the treatment protocol. Ideally, analgesic treatment of chronic pain should be mechanism-based. This means the mechanism of action of the chosen medication should target the pain mechanisms affecting that patient. For example, NSAIDS should be administered when there is predominantly inflammatory pain and antidepressants acting on serotonin pathways dispensed when there is predominantly pain disinhibition.
This approach has been advocated in people (Yarnitsky et al, 2012; Edwards et al, 2016) and some data also exist in cats with OA (Monteiro et al 2017, Guillot et al 2013).
The main challenge is that clinical pain is normally a mixture of different pain mechanisms rather than one acting in isolation. Therefore, clinically, individual treatment protocols are generally based on therapeutic trial. It is useful to inform owners that a period of trial and error should be anticipated before a definitive efficacious therapeutic plan that is ideal for the individual cat is found.
Additionally, owners should ideally be informed that this plan may need to be altered over time as the disease progresses. It is believed an analgesic agent should be administered for at least four weeks before deciding on treatment efficacy (provided no adverse effects occur). Drugs labelled for use in cats should be the first choice because of available safety and efficacy data. Additionally, they are generally more palatable and easier to administer in the long term.
NSAIDs
Currently, pharmacological treatment of pain centres around the use of NSAIDs. NSAIDs are indicated in the treatment of pain and inflammation associated with OA, as they produce both analgesic and anti-inflammatory effects (Lascelles et al, 2007). These are used to relieve pain and promote functional improvement (Sanderson et al, 2009).
Only two NSAIDs are approved for long-term use in cats, and then only in certain countries, so NSAID choice is limited in comparison to in dogs.
Meloxicam is a cyclooxygenase (COX)-2 preferential NSAID with high oral bioavailability, efficacy, palatability and good tolerability (Lascelles et al, 2007; Gunew et al, 2008; Guillot et al 2013). This compound seems to improve motor activity (Guillot et al, 2013), but not central sensitisation (Guillot et al, 2013; Monteiro et al, 2016).
Meloxicam is available in an injectable form, as well as tablets, an oral suspension and a transmucosal formulation. It is normally given at a loading dose of 0.1mg/kg once, followed by 0.05mg/kg by mouth every 24 hours. The efficacy of meloxicam is dose-dependent and administration of the minimum effective dose (0.01mg/kg to 0.03mg/kg) should be attempted if concerns exist with regards to potential adverse effects.
Robenacoxib is also a COX-2 preferential NSAID, available in injectable and tablet formulations. Robenacoxib appears to be well tolerated when administered daily for one month in cats with OA (King et al, 2016), but the efficacy of robenacoxib in cats with OA has not yet been reported. It is normally given at a dose of 1mg/kg to 2.4mg/kg by mouth every 24 hours. As with meloxicam, administration of the minimum effective dose should be attempted to decrease the risk of adverse side effects.
Although NSAIDs are routinely used to control pain and inflammation in cats with OA, safety concerns exist because of the high concurrent prevalence of chronic kidney disease (CKD) in this species and the paucity of data on the safety of these drugs in target clinical populations (King et al, 2016).
While it has been reported cats with OA commonly have concurrent CKD, causing them to be considered to be at increased risk for NSAID-related renal toxicity, no evidence actually exists to confirm that cats with CKD are at an increased risk (King et al, 2016).
A study assessed the safety of robenacoxib administration in 193 cats, including a subgroup of 40 cats with concurrent CKD (King et al, 2016). Safety endpoints included reports of adverse events, results of clinical examinations including bodyweight, and clinical chemistry and haematology variables.
In all 193 cats, including the subgroup with CKD, no differences existed in frequencies of reported adverse events, bodyweight change, or results of serum or urine chemistry or haematology variables. Therefore, robenacoxib appears to be well-tolerated in cats with evidence of concurrent CKD.
This data concurred with research data that showed no changes in renal function assessed by glomerular filtration rate and urine protein:creatinine ratio in normal and reduced renal mass cats when given meloxicam (Surdyk et al, 2013). In addition, in a previous clinical study, prolonged treatment with low dose meloxicam (0.02mg/kg/day) did not result in significant worsening of renal values in cats with CKD (Gowan et al, 2011).
Adjunctive analgesics and multimodal analgesia
Despite their widespread use and obvious benefit in many cases, NSAIDs are not always sufficiently effective when used as monotherapy (Lascelles et al, 2008). As a result of this, multimodal analgesia is commonly used in cats with OA. This implies the use of a combination of drugs that all act at different levels of the pain pathway and, therefore, have a synergistic effect, hopefully improving pain control and possibly allowing lower doses of individual drugs to be used.
Beyond COX-inhibiting NSAIDs, however, treatment options for the control of pain in cats are limited. Additionally, a paucity of evidence exists for the efficacy of these so-called adjunctive analgesics (Lascelles et al, 2008; KuKanich, 2013).
Amantadine, gabapentin and tramadol are the medications added to the treatment plan most commonly when treatment with an NSAID alone proves ineffective, and these are discussed in more detail below. Amitriptyline and codeine are alternatives, which may be used in challenging situations.
Tramadol
Tramadol is an analgesic used worldwide for its effects on improved physical function and good tolerability in humans with chronic OA-associated pain (Schaefert et al, 2015).
The analgesic effects of tramadol are expected to be mostly related to the production of its active metabolite(s) such as O-desmethyl tramadol (M1), which binds to µ-opioid receptors with approximately 300-fold higher affinity than the parent compound (Desmeules et al, 1996; Frink et al, 1996). However, µ-opioid receptor activation is only one of the mechanisms of action of tramadol and M1.
Other mechanisms of action include:
- norepinephrine and serotonin reuptake inhibition
- inhibition of G-protein coupled receptors such as α2-adrenoceptor, neurokinin 1 receptor and muscarinic receptor
- inhibition of ion channels via nicotinic acetylcholine receptor and N-methyl-D-aspartate receptor (Minami et al, 2015)
These mechanisms of action can increase the activity of the endogenous inhibitory control and decrease the pain transmission likely explaining the central analgesic effects of tramadol (Monteiro et al, 2017).
Recent evidence indicated that tramadol provided no clinical benefit to dogs with OA of the elbow or stifle joints (Budsberg et al, 2018). However, studies have indicated that cats might have a superior analgesic profile after tramadol administration when compared with dogs due to a longer elimination half-life and higher active concentrations of M1 (Pypendop et al, 2009; KuKanich and Papich, 2004).
Tramadol is a low-cost outpatient oral analgesic that appears to represent a viable option for the treatment of OA-associated pain in cats, including those with maladaptive pain (Monteiro et al, 2017). Side effects are uncommon, with the most commonly reported adverse events being mydriasis, sedation and euphoria. However, salivation may be observed after administration of tramadol due to the bitter taste, and treatment is unacceptable to some cats when palatability is an issue.
Forced pilling can impair the owner-cat bond and compromise feline welfare, so alternatives should be considered in these situations.
Additionally, tramadol should not be administered in combination with other serotoninergic drugs because of the risk of serotonin toxicity and some concern exists regarding an increased risk of gastrointestinal side effects when the tramadol-meloxicam combination is administered (Guillot et al, 2013).
Amantadine
Amantadine, as an N-methyl-D-aspartate (NMDA) antagonist, has been evaluated for its potential role as an analgesic (Bujak-Gizycka et al, 2012). The NMDA receptor and its ligand, glutamate, have long been implicated in the development and maintenance of central sensitisation, via increased and sustained excitation of neurons and subsequent alterations of gene and receptor expression (Latremoliere and Woolf, 2009; Baron et al, 2013).
Blockade of these receptors with NMDA antagonists has been shown to both prevent the development of central sensitisation, as well as treat the condition in affected animals (Wang et al, 2015; Tabakoff et al, 2016).
The use of amantadine in cats stems from anecdotal reports of efficacy (Robertson, 2008), and from demonstrated efficacy in dogs when used in conjunction with meloxicam (Lascelles et al, 2008). While the pharmacokinetics of amantadine, administered both IV and by mouth, have been documented in cats (Siao et al, 2011), clinical data demonstrating efficacy is currently lacking.
Amantadine should be used as an adjuvant drug in combination with other analgesics and owners should be aware that scientific information on its efficacy or safety for long-term treatment in cats is not yet available.
The current recommendation for dosing is between 3mg/kg to 5mg/kg once daily and in cats this generally necessitates use of the liquid formulation as capsule sizes are too large to allow appropriate dosing. As limited information is available regarding toxicity in cats, starting at the lowest dose and then increasing slowly as required based on response is recommended.
In humans, the adverse effects generally include minor CNS and gastrointestinal signs, but in cats amantadine appears to be well tolerated based on the limited information available.
Gabapentin
Gabapentin, an analogue of the neurotransmitter λ-aminobutyric acid, has been advocated for the treatment of neuropathic pain in cats because of experience treating neuropathic pain in humans (Backonja et al, 1998; Kukkar et al, 2013; Moore et al, 2014; Larsen et al, 2016).
Unlike many other analgesics, gabapentin has been reported to be efficacious in cats with OA. It decreased hypersensitivity in research cats with OA when administered at 10mg/kg every eight hours (Klinck et al, 2018c) and in client-owned cats, treatment with gabapentin resulted in improved owner-identified impaired activities when compared with placebo treatment (Guedes et al, 2018).
Because of the large safety margin and robust evidence of efficacy in human neuropathic pain, gabapentin is currently recommended in the management of feline chronic and neuropathic pain (Monteiro, 2020). Side effects are rare, but when they do occur generally involve mild sedation and ataxia, which often resolves with reduction of the dose.
Summary
In summary, the best treatment advice that we as vets may be able to communicate, is to expedite owner-education about feline OA and to encourage owners to remain actively engaged in the monitoring of their pet’s treatment.
Despite the high prevalence of OA in domestic cats, the condition remains underdiagnosed. When a diagnosis is reached, it is often late in the disease process, which may render the limited treatment options currently available less effective.
Additionally, determining the efficacy of a prescribed treatment for an individual cat remains challenging. As owner-reported behavioural signs remain the best assessment tool of feline chronic pain, education of owners regarding these signs, and more widespread use of clinical metrology instruments and other similar tools, may have the potential to facilitate diagnosis of OA in cats, allow treatment to be instigated earlier, and assist in tailoring of individual treatment protocols to maximise efficacy.
