6 Aug 2018
Canine lymphomas: diagnostic modality, therapy and innovation
Sara Verganti and Davide Berlato consider classification of lymphoma types in dogs and describe approaches to their diagnosis and treatment.
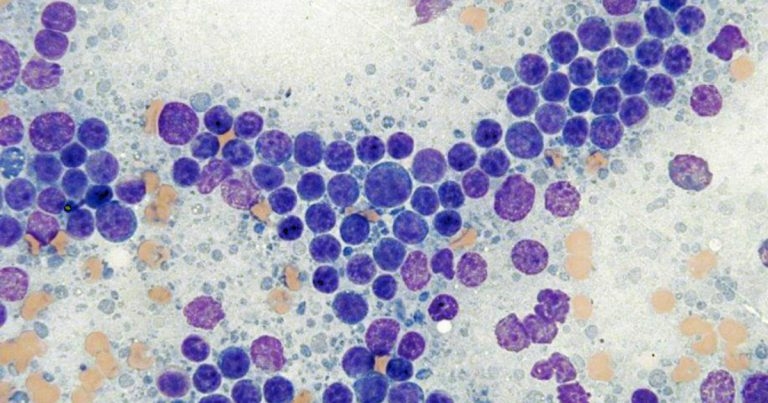
Figure 1. Cytological appearance of small cell lymphoma. Note the predominance of small lymphocytes with a mildly to moderately expanded population of small to medium lymphocytes. Rare large lymphocytes are seen. In this case, the diagnosis of lymphoma was confirmed via flow cytometry. Modified Wright stain 50× magnification. Image © Jennifer Stewart
The term lymphoma encompasses a heterogeneous group of neoplasms originating from the lymphoreticular cells. It is the most common lymphoproliferative disorder in dogs and one of the most common tumours seen in this species.
In recent years, significant changes have been seen in the veterinary oncology world in regards to classification, diagnosis and treatment of canine lymphomas, moving towards more type-specific and personalised treatments.
Canine lymphoma can no longer be considered a single disease entity. In fact, as in human medicine, numerous subtypes exist, with different responses to treatment and different prognoses.
According to the World Health Organization (WHO) classification system, 43 types of lymphoma exist, which can be classified based on a combination of clinical features, anatomic location, morphological characteristics, as well as immunophenotype and genetic features1-3. This has important clinical implications in terms of treatment decisions and the facilitation of clinical studies (that is, in assessing prognostic factors, efficacy of treatment and prognosis).
Most lymphomas in dogs are of B-cell origin (60% to 70%); however, marked breed variation in the prevalence of T-cell and B-cell types is also described4. The most common types of canine lymphoma are listed in Table 1.
| Table 1. The most common forms of lymphoma in dogs2,5,6 | |
|---|---|
| Aggressive forms | % of cases |
| Diffuse large B-cell lymphoma | 44-57 |
| Peripheral T-cell lymphoma not otherwise specified | 14-20 |
| Lymphoblastic lymphoma (mainly T, including hepatosplenic lymphoma | 3-5 |
| Burkitt-like lymphoma | 2 |
| Low-grade/indolent forms | % of cases |
| Marginal zone lymphoma (B-cell) | 6.5-16 |
| T-zone lymphoma | 3-12 |
| Small lymphocytic lymphoma | 5 |
| Follicular lymphoma (B-cell) | 1-5 |
In general, T-cell lymphoma is associated with a worse prognosis than B-cell lymphoma; however, it is more diverse in terms of cytological and histopathological characteristics, with unique immunophenotype and prognoses associated with some of the subtypes identified7.
Diagnosis
Multiple tests, used simultaneously or sequentially, are often required to achieve a definitive and accurate diagnosis of lymphoma, and help with prognosis. While cytology and histopathology used to be the main tests to diagnose lymphoma, additional tests have been developed, including immunocytochemistry/immunohistochemistry, flow cytometry, and PCR for the detection of clonal antigen receptor gene rearrangement (PARR)8.
Cytology
Fine needle aspiration/cytology is a quick, non-invasive and cost-effective method to assess the cell population of a lymph node or any other accessible organ (for example, liver, spleen or kidneys).
Based on cell morphology and cytological characteristics, the clinical pathologist is often able to differentiate between a reactive or neoplastic population. Nevertheless, the main limitations are the inability to assess the architecture of the tissue, making an appropriate classification difficult; the inability to sample deeply located lesions (for example, in the abdominal cavity); and the difficulties in differentiating reactive hyperplasia with early stages of lymphoma8.
Additionally, the diagnosis of small and intermediate-cell lymphoma can be challenging, requiring additional tests (for example, histology, immunohistochemistry, flow cytometry or PARR). In most cases, cytology has to be considered as an initial tool before further tests are performed.
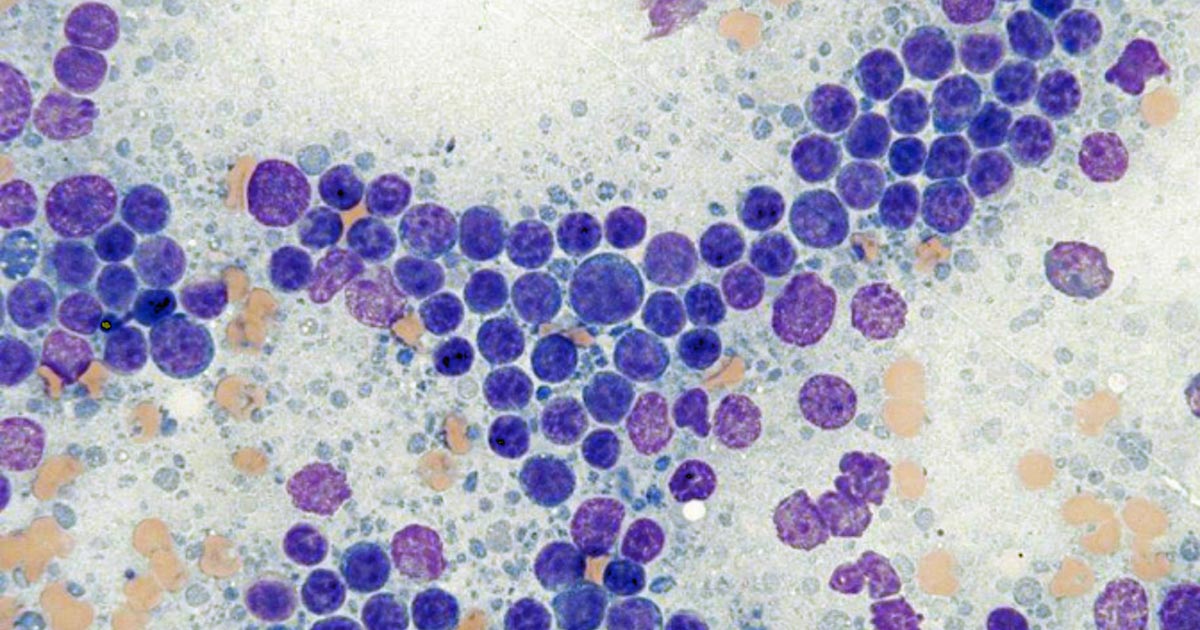
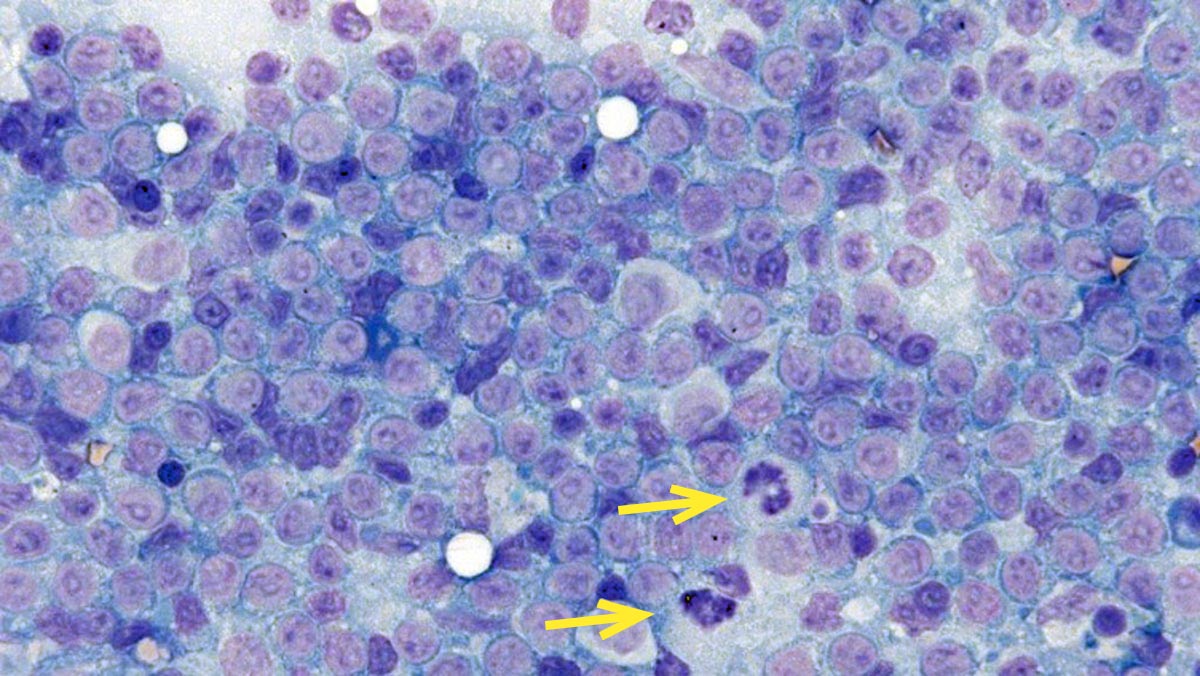
Figures 1 and 2 show the cytological appearance of a high-grade and low-grade lymphoma, respectively.
Flow cytometry
Samples for flow cytometry can be obtained from fine needle aspirates of solid organs – such as lymph nodes, spleen and liver – but also from fluids (for example, pleural and abdominal effusion or CSF), blood and bone marrow. Blood and bone marrow aspirate samples can be placed in ethylenediaminetetraacetic acid tubes, while samples from solid tissue should be placed in tubes containing buffered saline and a small amount of serum to help stabilise the cells before the analysis (contact the receiving laboratory for further information regarding specific submission guidelines).
The advantages are the sample can be obtained at the same time of obtaining samples for cytology and the turnaround is rapid (samples should be sent within 24 hours from collection and results are available within one to two days). It is important to submit – together with the sample for flow cytometry – slides for cytological evaluation of the sampled organ, blood or fluid. Compared to immunohistochemistry used on histological samples, a wider panel of antibodies can be used compared to histological samples to characterise the neoplastic population, allowing the identification of the immunophenotype and, in some occasions, the classification of the lymphoma8.
In addition, flow cytometry is able to assess whether a specific type of lymphoma is showing an aberrant expression of some specific markers (that is, expression of markers not usually expressed by non-neoplastic lymphocytes or not usually expressed by cells at that level of differentiation), which can be prognostic or may help in the diagnosis of lymphoma per se8,9. For example, a reduced or absent expression of major histocompatibility complex (MHC)II has been shown to be a negative prognostic indicator in B and T-cell lymphomas10,11, while the absence of cluster of differentiation (CD)45 in T-zone lymphoma (TZL) provides evidence of neoplasia12.
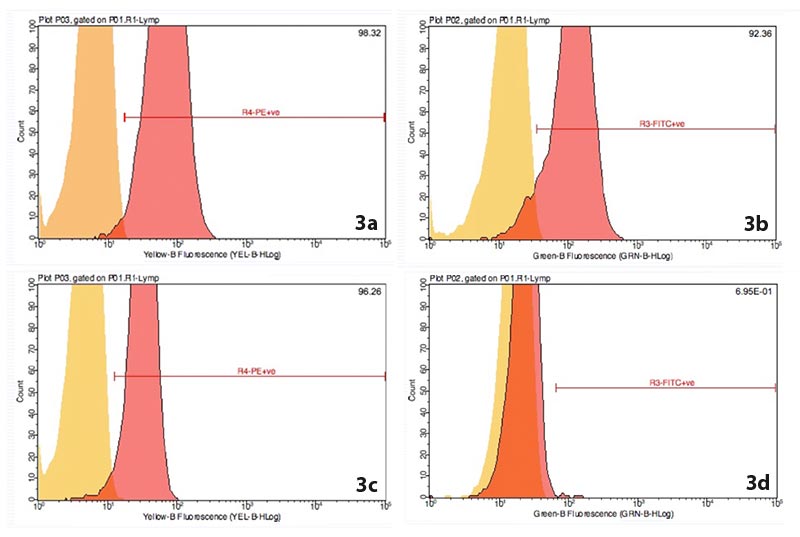
Flow cytometry is considered more sensitive than PARR in achieving a diagnosis of lymphoma13. Figure 3 shows part of the flow cytometry results for a dog diagnosed with diffuse large B-cell lymphoma.
Histopathology and immunohistochemistry
Histopathology can be sufficient for the diagnosis of lymphoma, allowing the identification of tissue architecture, morphological features and mitotic index. However, the diagnosis of specific subtypes of lymphoma, based on histopathology alone, is imprecise – therefore, immunohistochemistry becomes essential to accurately diagnose a lymphoma. Immunohistochemistry uses species-specific or species-limited antibodies that bind to cell antigens.
Compared to flow cytometry, the number of antibodies available is more limited, as formalin-fixed cells have altered expression of antigens compared to unfixed cells. When requested, several antibodies should be evaluated (for example, CD3 antigen for T-cell lymphosarcoma and CD79/paired box 5 for B-cell lymphosarcoma should be assessed simultaneously)8.
It is important to remember the lack of a positive result with immunohistochemistry does not rule out a specific tumour. In fact, negative results could be due to underfixation or overfixation of the sample, or loss of antigen expression by the tumour cells8.
Although incisional/tru-cut biopsies can be preferred as less invasive, excisional samples should be performed whenever possible to assess the overall lymph node architecture and to avoid false negative results (for example, in cases of early lymphoma or patchy distribution).
Compared to fine needle aspirates/cytology, this is a more invasive procedure that requires sedation or general anaesthesia. Processing times are also longer – especially if further tests such as immunohistochemistry or PARR analysis are performed.
PARR
PARR allows the identification of clonality in a B-cell and T-cell population. During their development, both B-cells and T-cells undergo rearrangements of the genes encoding the Ig receptor and the T-cell receptor (TCR), respectively. As a result, the TCR or Ig receptor differs in almost every lymphocyte8. Therefore, in a benign or reactive population, different PCR products will be present (polyclonal), while in neoplastic processes, a single product is present (monoclonal population).
The sensitivity and specificity of this test is high; however, false positive and false negative results can still occur. For example, oligoclonal or monoclonal population of lymphocytes can be seen in chronic infections, such as ehrlichiosis and Lyme disease, while a negative result does not rule out lymphoma8. Therefore, it is important to use PARR in conjunction with other tests, such as cytology, histology, immunohistochemistry and flow cytometry. The test can be run on blood or fluid samples, cytology slides (stained or unstained) and histology slides.
Treatment: is lymphoma a single disease?
High-grade B-cell vs T-cell lymphomas
The mainstay treatment for canine lymphoma remains systemic chemotherapy and multidrug protocols (for example, cyclophosphamide, vincristine and prednisolone [COP] or cyclophosphamide, doxorubicin, vincristine and prednisolone [CHOP]-based) have been proven to be superior to single agent protocols. For example, CHOP-based protocols – when used as first line treatment for high grade multicentric B-cell lymphoma – provide a median progression-free survival (PFS) of 6 to 10 months and a median overall survival of 10 to 12 months, compared to 6 to 9 months with a COP-based protocol and 1 to 2 months with prednisolone only14.
CHOP-based protocols are still the cornerstone treatment for high-grade B-cell lymphomas. However, high-grade multicentric T-cell lymphomas do not tend to respond as well as B-cell lymphomas, and dogs with this disease often achieve shorter responses. For this reason, in recent years, awareness has increased, supported by studies published in the literature that different protocols are required for T-cell high-grade lymphomas.
For example, a study evaluated the response in 41 dogs with newly diagnosed lymphoma a week after receiving a single dose of doxorubicin. For B-cell lymphomas, complete remission (CR) was achieved in 86% of the patients and partial remission in 14% of the dogs, for an overall response rate (ORR) of 100%. For T-cell lymphomas, only a minority of cases achieved CR (17%) for an ORR of just 50%15.
Four main protocols have been evaluated at this point for the treatment of high-grade T-cell lymphomas in dogs:
- L-asparaginase and CHOP (L-CHOP).
- L-asparaginase and mechlorethamine, vincristine, procarbazine and prednisolone (L-MOPP).
- Vincristine, L-asparaginase, cyclophosphamide, doxorubicin, prednisone-tuberous sclerosis complex (VELCAP-TSC).
- Lomustine, vincristine, procarbazine and prednisolone (LOPP)16-19.
Complete remission was achieved in 64% to 90% of dogs with an ORR of 73% to 97%. However, when looking into the specific protocols, CHOP-based treatments achieved the shortest response (Table 2). Based on this, T-cell lymphomas should be treated with protocols that incorporate drugs such as lomustine, rather than the standard CHOP-based treatments.
| Table 2. Comparison of four protocols for the treatment of high-grade T-cell lymphoma in dogs16-19 | |||||
|---|---|---|---|---|---|
| Protocol | Number of patients | Median PFS (95% CI) days | Median OST (95% CI) days | One-year survival rate % | Two-year survival rate % |
| L-CHOP | 24 | 104 (84-126) | 235 (164-244) | 14 | 5 |
| L-MOPP | 50 | 189 (99-278) | 270 (206-333) | ~35 | ~25 |
| VELCAP-TSC | 70 | 175 (119-231) | 237 (186-288) | 31 | 20 |
| LOPP | 31 | 176 (0-1,745) | 323 (51-1,758) | 39 | 25 |
| For details and definitions of each protocol, refer to the individual paper and main article. PFS = progression-free survival; CI = confidence interval; OST = overall survival time. |
|||||
Low-grade/indolent lymphomas
Low-grade/indolent lymphomas include different subtypes – such as marginal zone lymphoma (MZL), TZL, small lymphocytic lymphoma and follicular lymphoma – which originate from different organs or part of the lymph node, but share a clinical indolent course.
When indicated, chlorambucil/prednisolone is the treatment of choice. However, in some cases, treatment may be contraindicated. For example, the use of chemotherapy in TZL has been associated with decreased survival and a two to three-fold increased risk of death compared to lack of treatment. The use of prednisolone alone was associated with an increased risk of death of 25 times3,20.
If we consider another indolent lymphoma, studies have demonstrated splenic MZL is associated with a better outcome than nodal MZL. In fact, the reported median survival time for dogs with splenic MZL after surgery, with or without chemotherapy, is 383 to 939 days (1,153 days for non-symptomatic patients) versus 259 days for patients with nodal MZL treated with chemotherapy21-23. Based on this, further studies are required to assess the clinical course, the need and type of treatment, response to treatment, and prognosis of each specific subtype of lymphoma.
New lymphoma treatments
Rabacfosadine is a new drug shown to be effective against T-cell and B-cell lymphoma as well as multiple myeloma in dogs24. It is a double prodrug and exerts its cytotoxic activity by inhibiting different nuclear DNA polymerases. In a study, it was used alternately with doxorubicin in dogs with naive multicentric lymphoma, achieving an overall response rate of 84% and a median PFS of 194 days25. This might represent a valid alternative protocol to the standard CHOP-based chemotherapy as fewer visits are required; however, long-term follow-up data is still not available.
When used as rescue treatment for relapsed B-cell lymphomas, rabacfosadine appeared well-tolerated with an ORR of 74% (45% CR) and median PFS of 108 days26. Therefore, this can be considered a new possible rescue treatment, although the efficacy in heavily pre-treated patients has yet to be explored.
Immunotherapy is another exciting, but still emerging, treatment for canine lymphoma. In humans, monoclonal antibodies have revolutionised the treatment of B-cell malignancies. For example, rituximab, a chimeric monoclonal antibody against CD20, has become part of the standard treatment for chronic lymphocytic leukaemia, follicular lymphoma, diffuse large B-cell lymphoma and mantle cell lymphoma after several clinical trials have shown its efficacy in prolonging PFS and overall survival time27.
In veterinary medicine, many companies are working on the development of antibodies, but so far, none of the products developed have been effective.
- Please note some drugs mentioned in this article are used under the cascade.
