16 Dec 2019
Brigite Pedro and Chris Linney provide updates on the management of these issues, and ongoing research into control strategies and devices.

Image: Eric Isselée / Adobe Stock
With the publication of new American College of Veterinary Internal Medicine (ACVIM) consensus guidelines for the diagnosis and treatment of mitral valve disease (MVD)1, therapy and diagnostic recommendations have been updated from the initial and original 2009 consensus.
MVD accounts for up to 75% of all heart diseases seen in practice. Most dogs that develop a murmur secondary to MVD will do so years before the onset of congestive heart failure (CHF), for those that progress to CHF.
In one study, approximately 70% of dogs with asymptomatic MVD were still alive at the end of the 6.5-year observation period2. This means the majority of small-breed dogs with MVD either do not progress or progress slowly over years; therefore, management strategies and decision-making need to be stratified based on risk of progression.
The ACVIM consensus panel developed a staging system for MVD, which can be subdivided into those dogs that have asymptomatic MVD (stages A and B) and those with symptomatic MVD (with CHF; stages C and D). This sub-classification helps to further stratify the groups (Table 1).
| Table 1. American College of Veterinary Internal Medicine staging system for mitral valve disease (MVD) | |
|---|---|
| Stage | Description |
| Stage A | Dogs at high risk for developing heart disease, but have no identifiable signs of structural disease (for example, breeds predisposed to MVD, such as the cavalier King Charles spaniel). |
| Stage B | Dogs with structural heart disease (typical murmur with associated valvular changes), but have never developed clinical signs of heart failure. This stage can be further subdivided:
|
| Stage C | Symptomatic MVD dogs with either current or past clinical signs of CHF. Dogs may present as critical acute patients requiring hospital care, but may be able to be managed as an outpatient, dependent on the severity of clinical signs. |
| Stage D | Symptomatic, end-stage MVD patients with advanced CHF that is refractory to standard treatment. |
This article discusses the treatment options available at the various stages of MVD, and, where appropriate, provides a class recommendation (class I being the highest) and level of evidence (LOE; strong being the highest, weak or expert opinion considered lower level of evidence).
In MVD disease, however, a number of treatments are based on expert opinion in the absence of good clinical trials. Treatments with high recommendation class or strong level of evidence are summarised in Table 2.
| Table 2. Treatment recommendations for various American College of Veterinary Internal Medicine stages of mitral valve disease, with high level of recommendation or moderate-strong level of evidence to support use | |
|---|---|
| Stage | Treatment |
| Stage A | No treatment is recommended. |
| Stage B |
|
| Stage C | Acute (inpatient)
Chronic (outpatient)
|
| Stage D | Symptomatic, end-stage MVD patients with advanced CHF that is refractory to standard treatment. Acute (inpatient) As per stage C acute treatment, but also:
Chronic (outpatient) As per stage C initially, but the following treatments are recommended:
|
| *Low level of support for CRI. **Requires ICU monitoring. | |
Radiography may be beneficial (class I recommendation; LEO expert opinion) to assess for concurrent tracheal, bronchial and pulmonary disease.
Acquiring baseline radiographs may also allow clinicians to differentiate cardiac versus non-cardiac causes of future clinical signs, by having the patient’s previous radiographs for comparison.
Blood pressure should be considered in all patients and hypertension addressed if present, additionally to providing a baseline reading.
Echocardiography is recommended to fully assess the presence and severity of structural heart disease. Where echocardiography is unavailable, radiographs need to be interpreted with caution due to changes in thoracic conformation and breed differences in normal vertebral heart scales.
Recommendations for stage B1 management remain unchanged from the 2009 guidelines:
Stage B2 MVD dogs that meet the following criteria have been shown to benefit from therapy in large clinical trials4:
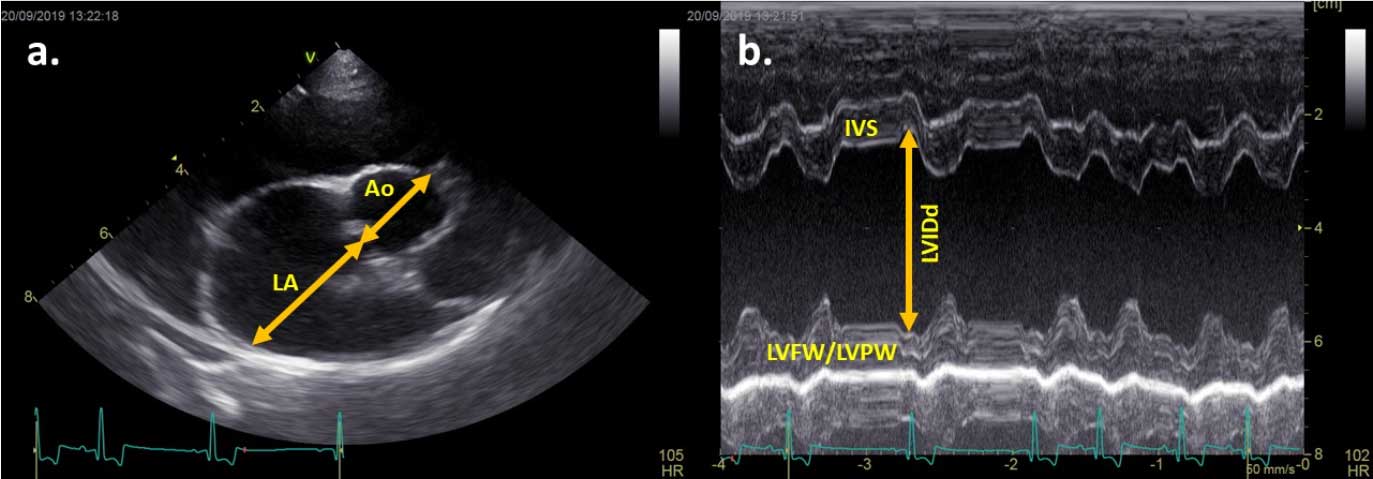
Ideally, all criteria should be met, but echocardiographic criteria provide the most reliable way to identify dogs where a treatment benefit is expected.
Where echocardiographic criteria is unavailable and radiography solely used, breed-adjusted VHS or a general-breed VHS greater than 11.5 could be used.
A new radiographic vertebral left atrial size measurement, with measurements greater than or equal to 3, is also likely to identify stage B2 MVD5.
Revised recommendations for stage B2 include:
The recommendation for the diagnosis of stage C MVD is the combination of echocardiography and radiography. The authors of this article also consider the use of lung ultrasound, a valid tool in an emergency setting where radiography is considered inappropriate if the patient is unstable6.
N-terminal pro-brain natriuretic peptide can also be considered; a normal or near-normal result in dogs demonstrating respiratory signs is highly likely to have a non-cardiac cause, rather than CHF, as the cause of clinical signs7.
Most dogs will be older patients, and assessment for comorbidities is recommended.
In the acute setting, for hospital-based treatment, a furosemide treatment plan of 2mg/kg IV (or IM), followed by a further 2mg/kg every hour until a substantial improvement in respiratory rate and/or effort is seen, to a total dose of 8mg/kg in 4 hours, is recommended (class I recommendation, LOE expert opinion). For life-threatening or peracute pulmonary oedema, a furosemide constant-rate infusion (CRI; 0.66mg/kg/hour to 1mg/kg/hour) after the initial bolus(es) could be considered (class IIa; LOE weak). Free access to water is recommended.
In the acute setting, pimobendan (0.25mg/kg to 0.3mg/kg orally or IV) is recommended to improve haemodynamic status and reduce afterload.
According to the guidelines, ACEis may be used in the acute setting; however, the authors withhold these in acute management due to a potential additive effect with furosemide administration (preload reduction), which may lead to acute kidney injury in the acute setting.
ACEis are commonly added at discharge if minimal azotaemia is present, or one to two weeks following clinical re-examination and review.
Optimal nursing care – including urination monitoring, access to water, maintaining an appropriate environment and positioning of sedated patients – are all beneficial.
Oxygen supplementation, if required, can be supplied via an oxygen cage or incubator, or via nasal oxygen cannulas. These environments can become hot and humid, so the environment ideally requires temperature and humidity control – or close monitoring of humidity and temperature at a minimum – both of which can confound tachypnoea and dyspnoea.
Sedation should be considered if anxiety associated with dyspnoea is present. Butorphanol is most often recommended (0.2mg/kg to 0.25mg/kg IM or IV), but other narcotics and sedatives can be considered.
With all agents, monitoring of blood pressure and respiratory response to narcotics is suggested.
Other considerations for stage C inpatient therapy include sodium nitroprusside for fulminant oedema, dobutamine for patients with cardiogenic shock non-responsive to pimobendan, glyceryl trinitrate and arteriodilators (although one must take care to monitor for hypotension and renal injury). As these require close and extensive monitoring of clinical parameters, these are options generally reserved for referral centres.
For chronic (home-based) treatment of stage C, quadruple therapy (spironolactone, pimobendan, ACEi and furosemide [or torasemide]; SPAF) is recommended at the onset of stage C. Chronic furosemide therapy for initial onset of stage C is typically 2mg/kg every 12 hours, but should be administered to effect.
The use of torasemide for first-line therapy is gaining popularity; doses of 0.1mg/kg to 0.3mg/kg have shown good success in managing early CHF8. Where oral furosemide dose reaches more than 8mg/kg/day when used in a SPAF protocol, patients are considered to be diuretic resistant and progress to stage D.
Measurement of urea, creatinine and electrolytes 3 to 14 days after starting or changing furosemide dose is recommended (class I; LOE weak). An ACEi (benazepril 0.5mg/kg every 12 to 24 hours) should be started at this time (if not already)9.
The authors may delay benazepril during initial acute management – for example, one to two weeks – where marked azotaemia and/or hypotension are present. Continue pimobendan (class I; LOE strong).
Spironolactone (2mg/kg every 12 to 24 hours) is recommended for its anti-aldosterone effects (class I; LOE moderate)10.
Beta blockers and nitroglycerin have limited to no indication in the chronic setting. Client home monitoring of respiratory rate, weight and appetite with home management is recommended (class I; LOE expert opinion).
Consideration of surgical intervention to repair the mitral valve apparatus in centres with low complication rates is encouraged where feasible (class I; LOE moderate).
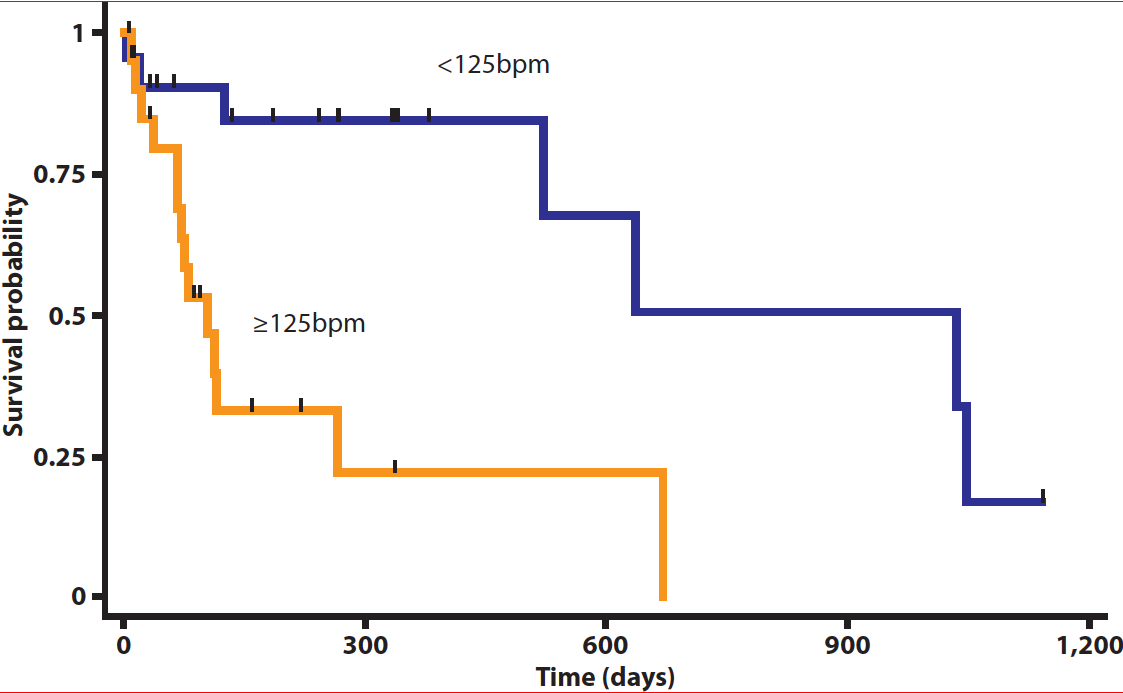
In cases with atrial fibrillation (AF), drugs to consider include the combination of diltiazem (0.5mg/kg to 2mg/kg modified release every 8 hours) and digoxin (2.5ug/kg to 5.0ug/kg every 12 hours) as first line therapy – a mean Holter ECG heart rate of lower than 125bpm was considered to show good control and a survival advantage (class I; LOE moderate)11.
Other considerations include cough suppressants and bronchodilators (class II; LOE expert opinion). The authors consider their use where CHF is under good control, but cough or respiratory signs are ongoing despite documented radiographic control of pulmonary oedema. These radiographs also help to exclude comorbidities.
Guidelines suggest moderate sodium restriction, monitoring urea, creatinine, and electrolytes (including magnesium, and both in late disease and if arrhythmias are present). Consider supplementation with omega-3 fatty acids if decreased appetite, muscle loss or arrhythmia occur (class IIa; LOE moderate).
Revised recommendations for stage D management include:
Stage D is diagnosed as per stage C, but with the addition of failure to respond to conventional SPAF protocol.
In an acute hospital-based setting, additional furosemide should be administered as IV boluses (2mg/kg every hour) for a maximum of four hours (class I; LOE expert opinion), or as a CRI (0.66mg/kg/hour to 1mg/kg/hour) until respiratory distress (rate and effort) has decreased. Alternatively, substitution for torasemide could be considered.
Cavity effusions should be removed to improve respiratory signs, where appropriate. If a patient can tolerate a reduction in afterload, arteriodilators should be considered. These can be given orally, but may require CRI administration IV, and referral to a cardiac or emergency and critical care unit is recommended by the authors if this option becomes necessary. Giving arteriodilators requires great caution as the combination of ACEis and arteriodilators can cause serious and life-threatening hypotension.
The benefits of afterload reduction outweigh the risk of hypotension in most cases, and in many cases “blood pressure reserve” exists to allow for titration to optimise the afterload reduction.
Other considerations are the management of pulmonary hypertension with sildenafil (1mg/kg to 2mg/kg orally every eight hours). Typically, this will be diagnosed with advanced echocardiography, but clinical signs – such as jugular distension or ascites with left-sided heart disease – may raise the suspicion for its presence.
Off-label use of pimobendan (for example, 0.3mg/kg every eight hours) could be considered (class IIa; LOE expert opinion).
For chronic management of stage D patients, higher doses of diuretics may be required. With higher dosing comes increased risk of clinically relevant azotaemia and electrolyte imbalance; these should be monitored closely.
All doses of the SPAF protocol should be optimised to their upper limits and all treatments recommended for stage C should be continued in stage D (except if furosemide is substituted for torasemide – then torasemide dose should be adjusted according to clinical requirements).
For chronic management, sildenafil, arteriodilators (amlodipine and hydralazine) and off-label pimobendan (every eight hours) should be considered.
Hydrochlorothiazide (or co-amilozide) was a recommendation by several panellists on the consensus committee, but using very low starting doses (0.5mg/kg to 2mg/kg orally every 12 to 24 hours), and titrating slowly due to the potential for acute renal injury and electrolyte imbalances.
Moderate sodium restriction and maintained calorie intake, along with omega-3 fatty acids, were recommended for dietary management.
AF is the most common non-physiological arrhythmia in dogs12,13. It is characterised by a fast and irregular rhythm on auscultation, accompanied by pulse deficits. Its diagnosis is easily made by ECG, where the absence of P waves – in combination with an irregular R-to-R interval, QRS complexes with normal morphology and fast heart rate – are pathognomonic.
Occasionally a normal rate may be observed. This so-called “lone AF” tends to affect large-breed to giant-breed dogs, where the atria are large enough to sustain the arrhythmia, similar to the horse14.
Most commonly, however, AF develops in association with an underlying heart disease that leads to marked atrial enlargement.
Unsurprisingly, the prevalence of AF is higher in older large-breed dogs12,13. An association between AF and dilated cardiomyopathy (DCM) is common – up to half of dogs with DCM are reported to also be affected by AF15,16. However, also, approximately 52% of dogs with myxomatous MVD weighing more than 15kg appear to be affected by this arrhythmia17.
AF can be an incidental finding in some dogs (particularly if lone AF); however, most patients with AF will have an underlying heart disease and clinical signs associated with CHF – including lethargy, generalised weakness, exercise intolerance, anorexia, weight loss, dyspnoea, tachypnoea, coughing or even syncope18.
AF also appears to contribute to the development of right-sided CHF; this was detected in almost 73% of dogs with DCM (versus 40% of dogs with DCM without AF) and 76% of dogs with degenerative MVD (versus 7% of dogs without AF)16.
Therefore, control of the heart rate is a more realistic aim. But a question remained until recently: what is the optimal heart rate for a dog with AF?
Recently, it was shown that in dogs with degenerative MVD, an ECG-derived heart rate lower than 160bpm was associated with longer survival time than a heart rate higher than 160bpm (171 days versus 61 days)17. However, a rate of 160bpm – or even 140bpm – is much higher than the heart rate of a normal dog23, and it is nowadays widely accepted these faster heart rates will contribute to atrial remodelling and heart failure24.
Another study has suggested dogs with a Holter-derived mean heart rate lower than 125bpm had longer survival time compared to dogs with a mean heart rate higher than 125bpm (1,037 days versus 105 days; Figure 2)11. The same study also showed for every 10bpm decrease in mean heart rate, the risk of all-cause mortality decreased by 29%11 – suggesting a clear benefit of lower heart rate within the normal sinus rhythm range.
An international multicentre prospective study is taking place to prospectively investigate the ideal rate control strategy.
Pulmonic stenosis (PS) and patent ductus arteriosus (PDA) are two of the most common congenital defects in dogs25; both conditions can be managed effectively with minimally invasive procedures where indicated.
Dogs with severe PS (transvalvular pressure gradient [PG] greater than 80mmHg) are at risk for developing clinical signs of exercise intolerance, syncope or sudden death26. Balloon valvuloplasty (BVP) is the recommended treatment in these cases, independently of clinical signs, or for any symptomatic dog with PS26.
According to literature, of the dogs that underwent a BVP, only the presence of clinical signs was significant to the outcome28; while of the dogs that did not have BVP, a higher PG, presence of tricuspid regurgitation, presence of clinical signs, valve morphology type B and younger age at diagnosis negatively affected survival28-30.
But how successful are BVPs? According to Locatelli et al27, the pre-BVP PG is the most important independent predictor of immediate and long-term outcome – the more severe the PS, the most likely the BVP results will be suboptimal.
This is particularly important in dogs with very high PG; it is not uncommon to have PS with a PG greater than 150mmHg – in these cases, achieving a PG of 50mmHg would be overly optimistic in many instances.
This was, in fact, the aim of a prospective study using high-pressure balloons (rated burst pressure between 12atm and 18atm, higher than the balloons routinely used in other studies)31. This type of balloon provided a median PG reduction of 63% and was considered successful in 92% of the cases (either by achieving a reduction in PG of at least 50% or a reduction into the moderate PS category with a PG lower than 80mmHg)31.
More recently, stent angioplasty has been shown to be a viable alternative for those rare cases with valvular PS where routine BVP is considered unsuccessful32.
However, when performed by an experienced operator, BVP tends to be a successful procedure for alleviating clinical signs and prolonging survival in dogs with severe PS18,26.
Complications are rare; however, a report has showed 7% of the patients undergoing BVP suffered major complications, including cardiopulmonary arrest, malignant ventricular tachycardia refractory to pharmacological treatment, cardiac perforation resulting in pericardial effusion, balloon rupture with pulmonary arterial fragment embolisation or jugular venous embolisation, and inability to access the right ventricle with concurrent tricuspid valve stenosis; although many centres – including that of the authors – have much lower complication rates than these reported33.
To avoid other major complications, it is also mandatory to rule out aberrant coronary arteries prior to a BVP, particularly in breeds where this anomaly has a higher prevalence – for example, bulldogs. These can be suspected based on echocardiography and diagnosed with an aortic root angiogram or CT angiography26,34,35.
In these cases, a conservative BVP may be performed; however, results tend to be suboptimal36.
A PDA is a vessel that connects the aorta to the pulmonary artery, allowing shunting of blood from the systemic to the pulmonary circulation, which, with time, may lead to volume overload of the left side of the heart.
The general recommendation is to close the PDA as soon as possible to avoid cardiac remodeling or even development of CHF – more than half of the dogs that do not undergo surgery will die within one year37,38; when the PDA is closed early in life, animals can have a normal life expectancy39. Many devices have been developed over time for minimally invasive PDA closure, with transvenous or transarterial approaches. Coils appear to have been largely replaced by Amplatz canine duct occluder (ACDO) devices37,40.
Nowadays, coils are mainly reserved for very small patients – their smaller profile allows access through small-calibre vessels in smaller patients. However, the risk of dislodgement or incomplete occlusion makes the ACDO a much more attractive option40.
In the attempt to override these limitations, new devices are being explored – low-profile ACDOs and Amplatzer vascular plugs (AVPs)39,41. In the authors’ practice, human devices – such as a type-two AVP – have also been routinely used (unpublished data; Figure 3).
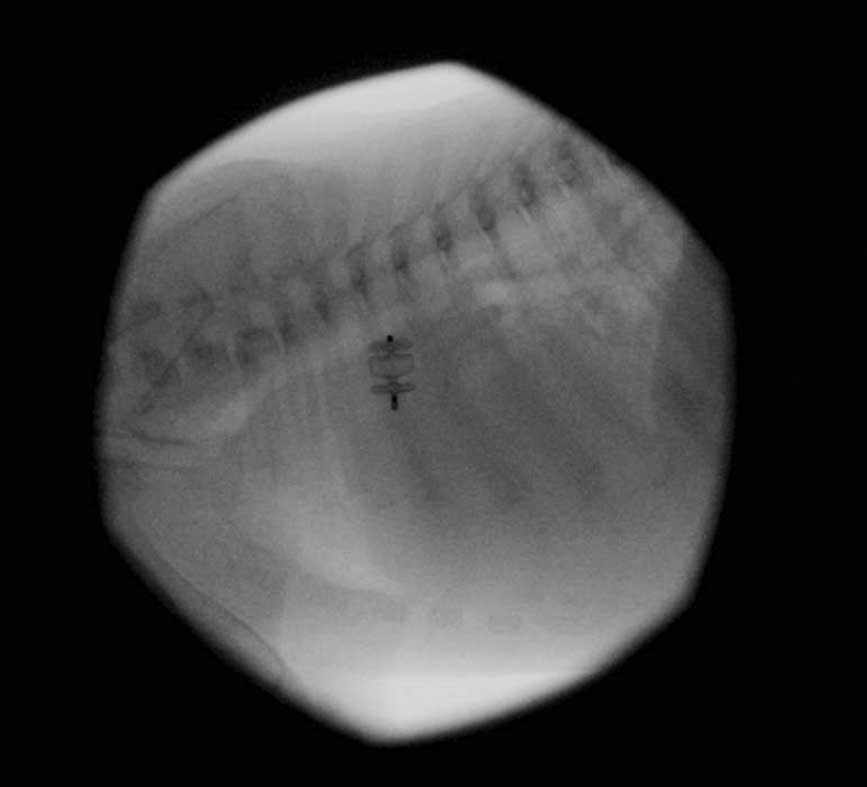
Additionally to the challenge the initial approach can represent due to the dimensions of the vascular access, correct sizing of the PDA ostium (or ampulla, depending on the type of device to be used) is another vital step.
It is important to use more than one modality to evaluate the size of the ostium and/or ampulla, as this is essential to guide the selection of the right size device. Unfortunately, it has been shown not even bodyweight can help predict the size of the PDA ostium42; therefore, a good imaging technique is required.
The agreement between transthoracic echocardiography, transoesophageal echocardiography, angiography and 3D echocardiography is suboptimal; therefore, the modality that allows the best visualisation of the PDA for each case should be used for measurements and/or to guide the procedure43.
In rare occasions, surgical ligation of the PDA is an alternative to consider – this decision is mainly based on patient size, availability of different techniques in the practice and, potentially, costs.
Similar success rates have been reported between surgical ligation and minimally invasive occlusion of the PDA; however, the latter is associated with fewer complications33,44.
Embolisation of the device is one of the most reported – although rare – complications and is the main reason why temporary exercise restriction is recommended in the immediate postoperative period33,44.
The diagnosis and consequent closure of a PDA tends to occur at a young age, when the loud continuous murmur is detected. However, in some cases, this can be missed and a PDA is, therefore, identified in older dogs. This is usually associated with cardiomegaly and clinical signs of CHF, which would still benefit from closure – even if other concurrent cardiac diseases are present45.