25 Mar 2025
Chronic kidney disease in cats – management options beyond diet
Kit Sturgess MA, VetMB, PhD, CertVR, DSAM, CertVC, FRCVS considers the most common issues faced by felines with this condition, and the drugs most suitable for treating and managing them.

Figure 1. A dehydrated, inappetent patient with International Renal Interest Society stage 3 chronic kidney disease.
While diagnosis of chronic kidney disease (CKD; Figure 1) is relatively straightforward in cats, it is not a homogeneous disease.
The kidney has many functions beyond excretion of nitrogenous waste, including fluid balance, acid base balance, regulation of blood pressure and the production, excretion and metabolism of hormones.
Due to this complexity, and the fact that the diagnosis is for the rest of the patient’s life, characterising all the facets of a specific individual’s CKD is important to develop an appropriate care package. It is also important for the carer to understand that CKD is a dynamic disease and, therefore, that care package may need to change over time.
The challenge for the clinician caring for the patient is a wide range of potential medications could be prescribed, and they need to choose options that are appropriate and achievable, both from an owner and a patient perspective, to maximise quality of life and longevity.
When creating a care package, it is critical that the carer’s ability to administer the medication long term, and the financial impacts of treatment and monitoring, are considered.
Diet remains the mainstay of initial treatment. The recommendations outlined by the International Renal Interest Society (IRIS) should form the basis of any plan, and this is the starting place for a care package. Nutrition can, to a greater or lesser extent, address many of the issues that CKD cats have, but as the disease progresses diet alone is rarely sufficient.
For the majority of carers, quality of life is the key issue, with inappetence and loss of body condition being a major stressor for them, and a significant driver towards a decision to euthanise.
A good care package is not about a formulaic approach, but about asking yourself key questions, such as:
- Although we have dietary therapy in place, is calorie intake sufficient? If not, do I need to be managing nausea or inappetence to maintain body condition?
- When should treatment start, and how do I rank them in context with other treatment goals?
- My patient is hypertensive; what is the best approach to treatment?
- If proteinuria is present, what is my approach to managing this?
- Is my patient anaemic? If so, is it at a level that requires intervention?
- What options are available to manage hyperphosphataemia beyond diet?
- Is treatment becoming too complex for the carer and impossible to administer to the patient, damaging the carer-cat bond and leading to an unacceptable caregiver’s burden?
- What about managing calcium, potassium and acidosis?
- Does a place exist for other therapies such as mesenchymal stem cell therapy?
- How can I manage my patient’s concomitant comorbidities; for example, osteoarthritis pain? How does managing these other conditions impact the plan for the patient’s CKD?
It is outside the scope of this article to discuss in detail every possible treatment option and combination, so the focus will be on:
- Managing body condition and nausea.
- Managing hypertension and proteinuria.
- Managing hyperphosphataemia and hypokalaemia.
- Approach to anaemia.
Managing body condition and nausea
Ensuring adequate calorie intake is critical for long-term well-being of CKD cats. Loss of muscle mass also reduces the body’s nitrogen excretion capacity.
The poor appetite that many CKD cats have (Figure 2) is likely to be multifactorial, including learned taste aversion, poor hydration status, gastric fibrosis and calcification, and the impact of uraemic toxins on the chemoreceptor trigger zone. Antiemetics may have a beneficial effect, and many cats will respond to the chronic use of maropitant, although the author has often found ondansetron to be more effective. Ensuring adequate hydration may also help.
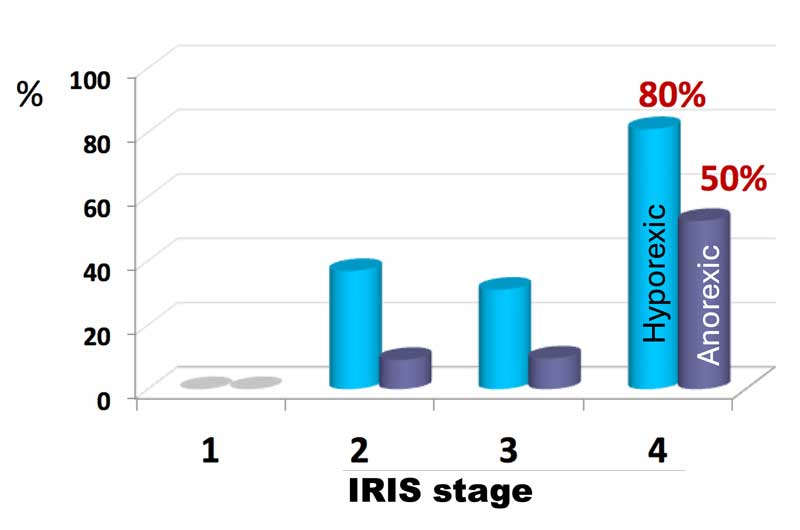
For some cats, to maintain body condition, the chronic use of appetite stimulants is necessary.
Over the years, a variety of drugs have been used, including B12 and anabolic steroids, corticosteroids, benzodiazepines, cyproheptadine and mirtazapine. In the UK, mirtazapine is the most widely used drug and comes in a variety of formulations, including transdermal cream. Published studies have shown increased food intake, but the effect is quite variable between cats.
More recently, ghrelin receptor agonists are becoming available. Ghrelin is a peptide hormone produced in the stomach in response to fasting. It acts in the hypothalamus, pituitary and other organs, stimulating appetite and enhancing the release of growth hormone and insulin-like growth factor 1, resulting in increased muscle mass and reduced glycaemic variability.
Capromorelin is available in the US and an increasing number of countries in Europe as a once-daily liquid medication. In healthy cats (Wofford et al, 2018), increased bodyweight compared to controls is reported. In licensing studies in CKD cats, 84% of cats receiving capromorelin maintained or gained bodyweight compared to 41% of controls.
Transient hypersalivation is a relatively common side effect, occurring in one out of five of cats.
Managing hypertension and proteinuria
Proteinuria in CKD is relatively common. In cats, the IRIS classification is based on using urine protein to creatinine ratio (UPCR), which gives an estimate of proteinuria.
It is important to realise that UPCR varies day to day, by methodology, by laboratory and by sample collection method. A UPCR of less than 0.2 is considered within reference, 0.2 to 0.4 as equivocal, and greater than 0.4 as evidence of proteinuria. Those cats with the UPCR consistently greater than 0.4 should be treated.
First choice therapy would be with either an angiotensin-converting enzyme inhibitor such as benazepril, or with an angiotensin receptor blocker such as telmisartan. No clear guidelines exist as to which to use first. If proteinuria is non-responsive to a maximum dose of the first-choice drug, then the alternative can be trialled. If neither is effective alone, then both drugs can be used together.
Some evidence also exists showing that using aldosterone antagonists such as spironolactone may be beneficial, as high levels of circulating aldosterone have been associated with reduced survival time and aldosterone antagonists reduce proteinuria in man and in a small study in cats (Kai et al, 2022).
Wherever possible, blood pressure should be monitored in cats with CKD. The American College of Veterinary Internal Medicine has produced a consensus statement on the management of hypertension (Figure 3).
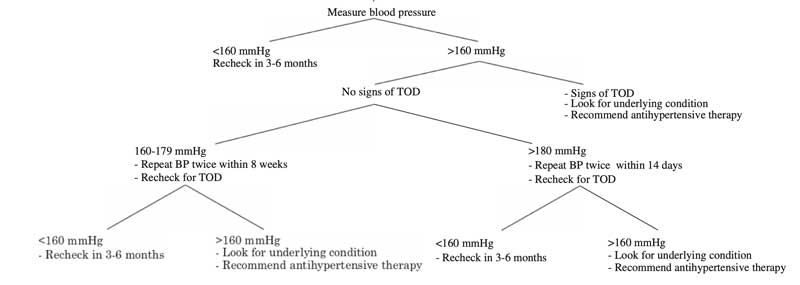
First-line treatment of hypertension in cats is with amlodipine, a calcium channel blocker, or telmisartan, an angiotensin receptor blocker. Both drugs have been shown to result in a significant reduction in blood pressure in about 70% of cats. No studies exist that directly compare amlodipine with telmisartan; therefore, the choice of which drug to use is down to the individual clinician.
Studies on amlodipine have not shown that its use increases survival time; similar studies for telmisartan have not been undertaken, but telmisartan more directly affects the mechanisms that may be leading to hypertension. As telmisartan also reduces proteinuria, it would be the author’s first choice in hypertensive proteinuric CKD.
Whichever drug is used, it should be started at an appropriate dose depending on the level of hypertension and target organ damage, and increased until it is effective or the maximum recommended dose is reached.
The full effect of the dose change for amlodipine takes five to six days to become evident. Telmisartan reaches a maximum effect more quickly.
If the first-choice drug is ineffective, then the options are to replace with an alternative, but a risk exists of temporarily worsening hypertension; where appropriate, introducing a second drug is probably a safer strategy.
Managing hyperphosphataemia and hypokalaemia
Hyperphosphataemia
Diet would be the first approach to managing hyperphosphataemia, but some cats will not accept a dietary approach, and some cats have more advanced CKD where diet alone is insufficient.
If phosphate is to be controlled, then phosphate binders need to be used, which adds additional medication to the care plan. However, managing phosphate has been shown to have a major impact on survival time and quality of life (Figure 4).
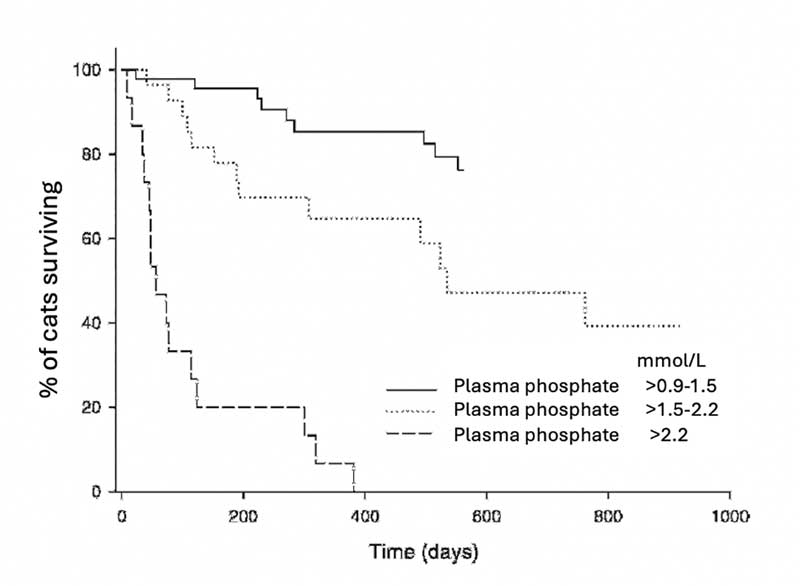
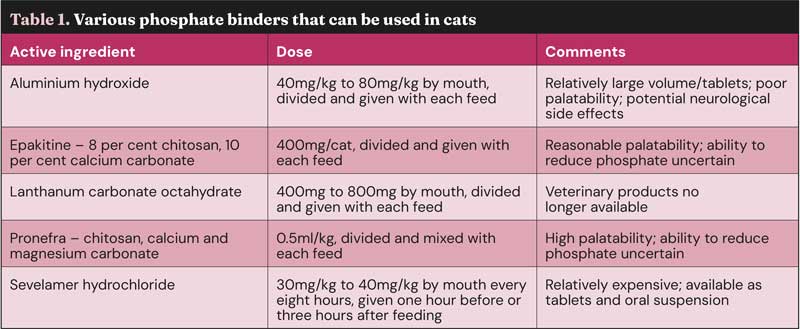
Deciding which phosphate binder to use (Table 1) is a balance between efficacy, frequency, volume of administration and palatability. Unfortunately, the more palatable binders tend to be less effective, and concerns exist about using aluminium-based products. Sucralfate has been suggested as an option, but published studies do not show it has an impact on phosphate levels. Phosphate binders need to be given with food, so are a challenge in cats that are ad lib – especially dry-diet fed. Sevelamer or lanthanum products provide the best overall balance, and would be the author’s first choice where diet has failed, and blood phosphate is sitting outside the level recommended in the IRIS staging.
It is important to remember that cell lysis will significantly increase phosphate levels, so phosphate levels should not be measured if the sample is haemolysed.
Hypokalaemia
Potassium is wasted to try to maintain circulating volume and acid-base balance; potassium loss is higher in acidotic patients.
Significant hypokalaemia causes an inflammatory myopathy, initially seen as a stiff, stilted gait and is easily mistaken for osteoarthritis in older cats. Hypokalaemia may also lead to increased ammoniagenesis, which results in more rapid progression of CKD.
Commercial renal diets are potassium supplemented, and it is important to appreciate that the dietary potassium requirement is dependent on the protein level in the diet; the higher the protein, the more potassium is required. As most potassium is intracellular, assessing whole body potassium deficiency can be challenging. If serum potassium is consistently low, then supplementation is indicated; whether supplementation earlier than this is beneficial is unknown.
Measuring urinary fractional excretion of potassium will give some indication of the levels of loss.
Potassium gluconate is the most widely used potassium supplement in cats, with most cats requiring 2mmol to 4mmol (mEq) per day. In acidotic patients, potassium bicarbonate can be considered. Effervescent potassium supplements for people are most commonly potassium chloride and should be avoided, as they are acidifying.
Approach to anaemia
Many CKD cats are mildly anaemic, which will occur for a variety of reasons (Panel 1), but does not need treatment. CKD cats with moderate to severe anaemia (haematocrit less than 18%) are likely to benefit from intervention to increase their red cell numbers.
Deciding the underlying cause of the anaemia can be difficult and in many patients likely multifactorial. Initial efforts should be to ensure adequate nutrition and iron status, minimise uraemic toxin levels, manage intestinal bleeding if present and manage other comorbidities that may be contributing to an inflammatory state (anaemia of chronic disease). If the anaemia persists, erythropoietin levels can be measured to determine whether supplementation is likely to be effective.
In the past, anabolic steroids have been used which do have some impact on red cell production, but this is usually insufficient. Injections of erythropoietin or analogues can be highly effective, but are relatively expensive.
Analogues such as darbepoetin (Panel 2) are usually preferred, as they can be given less frequently and have a lower risk of the patient developing an immune response to the drug. Chalhoub et al (2012) showed markedly improved survival in cats whose anaemia responded to darbepoetin therapy.
Renal hypoxia is the main stimulus for the production of erythropoietin by the juxtaglomerular apparatus, with this process being mediated by hypoxia-inducible factor (HIF; Figure 5).
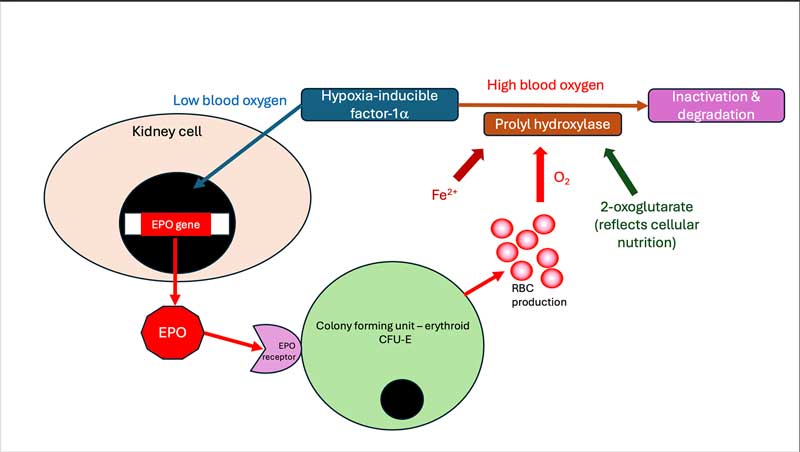
Molidustat is an oral drug which acts to inhibit the enzyme that degrades HIF, and has been shown to increase the red cell count in 7 out of 14 cats with non-regenerative anaemia and CKD (Charles et al, 2024). Vomiting associated with dosing was reported in 40% of cats. Despite this, these drugs have the potential to significantly improve the quality of life and longevity of anaemic CKD cats.
Conclusions
This article has not considered all possible treatment options for managing CKD in cats, but focused on the most common issues that affect a patient’s quality of life and longevity.
CKD in cats needs the clinical team to develop an individualised treatment plan, ensuring the delivery of contextualised care.
To achieve this, a thorough understanding of the impact of a specific patient’s CKD is essential, so that a care plan can be developed targeting the key issues that are likely to be affecting quality of life and longevity while taking into account the carer’s ability to deliver the treatments, as well as cost consideration of the treatment and monitoring that is required.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
- This article appeared in Vet Times (2025), Volume 55, Issue 12, Pages 6-10

References
- Acierno MJ, Brown S, Coleman AE, Jepson RE, Papich M, Stepien RL and Syme HM (2018). ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats, J Vet Intern Med 32(6): 1,803-1,822.
- Chalhoub S, Langston CE and Farrelly J (2012). The use of darbepoetin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases, J Vet Intern Med 26(2): 363-369.
- Charles S, Süssenberger R, Settje T, Langston C and Lainesse C (2024). Use of molidustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor, in chronic kidney disease-associated anemia in cats, J Vet Intern Med 38(1): 197-204.
- Kai M, Ohishi T and Hikasa Y (2022). Effects of plasma aldosterone concentration and treatment with eplerenone on the survival of cats with chronic kidney disease, Can Vet J 63(12): 1,226-1,235.
- Wofford JA, Zollers B, Rhodes L, Bell M and Heinen E (2018). Evaluation of the safety of daily administration of capromorelin in cats, J Vet Pharmacol Ther 41(2): 324-333.
