18 Sept 2020
Jo Murrell discusses diagnosis and treatment of this issue, including the use of clinical metrology instruments to quantify it.
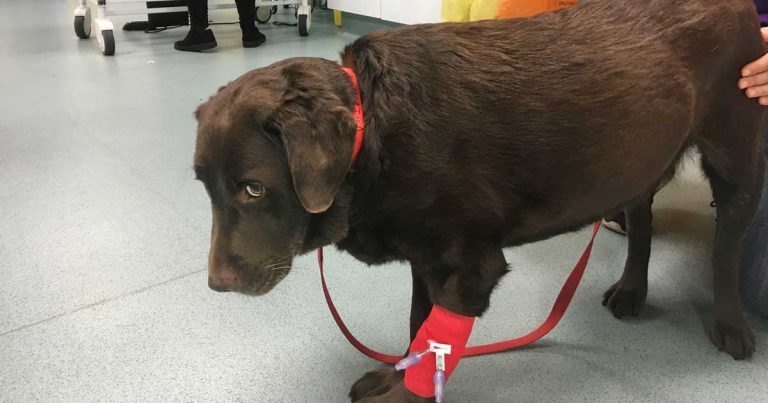
Figure 1. A dog with profound neck pain. Note that it would be preferable to use a harness instead of a lead in these cases.
The International Association for the Study of Pain (IASP) recently defined chronic pain in humans as pain that persists or recurs for more than three months (Scholz et al, 2019).
This definition may be relevant to veterinary medicine, although it should be considered that due to the shorter lifespan of cats and dogs, compared to humans, the three-month time span may be too long.
The IASP also makes a distinction between chronic primary pain, where the chronic pain is perceived as a disease in its own right – for example, in fibromyalgia – and chronic secondary pain, where pain is secondary to an underlying disease. Examples here include chronic secondary musculoskeletal pain.
Bell et al (2014) investigated veterinarians’ attitudes to chronic pain in dogs. Common causes of chronic pain in dogs were OA, and aural and dental disease; although spinal and skin disease, and neoplasia were also mentioned.
Major barriers associated with treating chronic pain in dogs were compliance, assessment of chronic pain and expense, and a wide range of drugs were used to treat chronic pain in dogs.
A recent small qualitative study (Davis et al, 2019) investigated owner perceptions of chronic pain in their dogs and found chronic pain in them had a very significant impact on owners. Owners were particularly concerned about detecting when their dogs were in pain, and worries existed about the expense of treating chronic pain and having to implement changes in routine to accommodate administration of medications.
This illustrates that implementing tools, such as clinical metrology instruments, to assess chronic pain in dogs may be very well accepted by owners and will address one of their main concerns.
Diagnosing and managing chronic pain in companion animals is important. Pain has a global impact on an animal’s well-being and quality of life. It can cause changes in mobility, willingness to play and mood, with many owners reporting their pet in chronic pain has a low mood or mood swings.
Therefore, it is important to assess both the sensory and emotional components of chronic pain, and monitor changes in both aspects when starting treatment with analgesics.
Diagnosing chronic pain in dogs and cats is challenging, and takes time. We are primarily reliant on behavioural changes to detect chronic pain – and in this respect pet owners are a key determinant in reporting these behavioural changes to the veterinary surgeon.
It is important to listen and question owners about the behaviour of their pets, to ascertain changes in behaviour that may be linked to pain.
One key differential when discussing behavioural changes is differentiating between changes due to pain and changes due to the ageing process, as some chronic pain conditions – such as OA – tend to be more prevalent in aged animals. An analgesia trial may help to distinguish between the two.
Chronic pain is maladaptive (Adrian et al, 2017) and, as such, is associated with findings on clinical examination, such as hyperalgesia and allodynia.
Hyperalgesia is defined as increased pain from a stimulus that normally provokes pain, and can be detected by applying firm pressure around a wound or painful area and ascertaining whether an exaggerated pain response occurs.
Allodynia is pain due to a stimulus that does not normally provoke pain, such as light touch, with the stimulus leading to an unexpectedly painful response.
It is important to try to measure both the sensory and emotional impact of chronic pain on an animal. The sensory impact is easier to ascertain and can be monitored using clinical examination, and trying to measure the magnitude of allodynia and hyperalgesia.
Determining the emotional status of a cat or dog is much more challenging; asking the owner questions about play, and behaviours the animal chooses to do when it is not painful, can be helpful.
It can also be useful to try to distinguish between different underlying mechanisms of pain to facilitate decisions around treatment. Probably the most important distinction is between neuropathic type pain, where the CNS or peripheral nervous system is damaged; functional pain, where no changes exist in the structure of the nervous system, but the functioning of the nervous system is abnormal; and inflammatory pain, which is adaptive and serves as an early warning system to prevent further tissue injury (Adrian et al, 2017).
Drugs such as gabapentin or pregabalin may be more appropriate to treat neuropathic and functional type pain rather than inflammatory type pain. However it is also important to be aware that many chronic pain conditions may have mixed aetiologies – for example, advanced OA may have both an inflammatory and neuropathic component.
Very recently, Enomoto et al (2020) developed a checklist to diagnose degenerative joint disease (DJD) in cats. The study used data collected during previous studies of DJD in client‑owned cats to generate the six‑question binary checklist in a data‑driven approach. The checklist is simple to use and has the aims of allowing the veterinary surgeon to screen for DJD in a clinically expedient manner, as well as providing a foundation for increasing awareness of DJD among cat owners.
The questions in the checklist are listed in Table 1. If no is answered to any question, it should prompt further evaluation of the cat for DJD (for example, orthopaedic assessment and radiographs).
| Table 1. Questions in the checklist to help diagnose degenerative joint disease in cats | ||
|---|---|---|
| Question | Yes | No |
| Does your cat jump up normally? | ||
| Does your cat jump down normally? | ||
| Does your cat climb up stairs or steps normally? | ||
| Does your cat climb down stairs or steps normally? | ||
| Does your cat run normally? | ||
| Does your cat chase moving objects (toys, prey and so on)? | ||
The Canine Osteoarthritis Staging Tool (COAST) has recently been developed to help stage OA in dogs (Cachon et al, 2018) and, therefore, facilitate decision‑making about treatment. It has a number of different stages, including an assessment of the dog (using a clinical metrology instrument), assessment of mobility and pain, and grading of the affected joint (selecting the most severely affected joint), including radiography. These outcome measures are used to determine the grade of OA according to the COAST tool.
The interactive COAST PDF is available online.
The most useful tools for quantifying chronic pain in dogs (Figure 1) and cats are clinical metrology instruments, which mostly comprise owner questionnaires. This reflects the fact owners spend more time with their pets than veterinary surgeons and are, therefore, better able to gauge how chronic pain is impacting on their pet’s life and best placed to monitor response to treatment.
However, owner education about chronic pain and signs to look out for in their pets is also the key to success of this approach.
A number of published and validated clinical metrology instruments exist to assess chronic pain in dogs and cats, although many have a focus on OA‑mediated pain.
For example, the Liverpool Osteoarthritis in Dogs (LOAD; Walton et al, 2013) questionnaire is a 13‑item clinical metrology instrument used to assess canine articular disorders such as OA. Individual question scores are summed to provide an overall LOAD score suggestive of disease presence and severity. It is freely downloadable along with instructions about how to administer the questionnaire in clinical practice.
The Canine Brief Pain Inventory (CBPI; Brown et al, 2008) is another owner questionnaire that can be used to assess chronic pain in dogs. It was initially developed to quantify pain in dogs with bone cancer, but has also been validated to measure pain associated with OA. It is divided into two sections, with four questions about pain severity and six questions about pain interference with daily activities, and a final question about quality of life. The mean score for pain severity and the mean score for pain interference are used to calculate the CBPI scores, and changes in these scores can be used to monitor response to treatment.
No owner questionnaires have been specifically designed to quantify neuropathic pain in dogs, such as pain associated with syringomyelia, although it has been suggested monitoring of phantom scratching behaviour may be a good surrogate measure of pain and quality of life in these cases (Hechler and Moore, 2018).
Until recently, an owner questionnaire to quantify pain associated with OA was lacking in cats (Figure 2); this has been rectified by the development of the Feline Musculoskeletal Pain Index (FMPI; Benito et al, 2013a; 2013b). The index is freely downloadable.
The FMPI comprises 18 questions relating to a cat’s mobility (walking, jumping up, jumping down and climbing stairs) and behaviours that may be more indicative of quality of life – for example, interaction with other cats and family members, play-related behaviours and grooming.

Each question has seven possible responses – ranging from “above normal” to “not at all” or “doesn’t apply or I don’t know” – and the owner has to tick the most appropriate response. No numerical score is applied to any of the possible answers so that an owner’s responses are not skewed by a value being assigned to any one possible response.
One potential disadvantage of the index is that it relies on the owner being aware of what is “normal” for a cat, which may be difficult for some owners and skew the results, although the authors of the index do not report it to be problematic.
Three additional questions relate specifically to pain and quality of life:
Asking owners to directly consider their pet’s quality of life can be very helpful in decision‑making about the optimal time to implement analgesic therapy, change analgesic therapy or even consider euthanasia.
To analyse a completed index, the authors have (post‑completion) assigned scores to the different possible answers – ranging from -1 (above normal) to 4 (not at all) for activity-type questions. The pain and quality of life questions have answers ranging from 0 (no pain or excellent quality of life) to 4 (severe pain or poor quality of life). This gives a total possible score of 83 corresponding to maximum impairment or pain. To date, no intervention criterion where administration of analgesics is indicated has been determined.
The FMPI has been shown to discriminate between normal cats and cats with pain caused by OA (Benito et al, 2013b), and showed good test-retest reliability when owners were asked to complete the questionnaire twice, two weeks apart (Benito et al, 2013b).
However, somewhat surprisingly, the index did not perform well in a study designed to investigate the responsiveness of the index (whether the instrument could detect a change in OA-related pain because of treatment; Benito et al, 2013a), suggesting that some further refinement of the scale is needed.
The research group at the University of Montreal has developed two tools for assessment of OA in cats – one for the caregiver and one for the veterinarian (Klinck et al, 2018).
The Montreal Instrument for Cat Arthritis Testing (MI-CAT) tool for the caregiver was developed in a similar manner to the FMPI scale. The latest version of the scale comprises 18 items in subscale one and 20 items in subscale two. Each item is scored as a yes or no relating to the cat’s ability to perform a certain activity, and a final score is calculated.
The MI-CAT tool for veterinarians was developed for use in cats with OA and comprises 25 items relating to body posture, gait, willingness and ease of horizontal movements, jumping and a general lameness score.
Preliminary validation showed the scale could distinguish between cats with OA and healthy control cats, but that it was unable to discriminate an effect of treatment.
Chronic pain is very common in dogs and cats, and has a global impact on an animal’s well‑being and quality of life; as such, diagnosis and treatment of chronic pain is pivotal to animal welfare.
A number of validated tools can quantify chronic pain in cats and dogs, and can be used to obtain a baseline sense of the chronic pain state and monitor response to drug therapy.
A reasonably wide range of drugs are used to treat chronic pain, but many are unlicensed and lack supportive data from clinical studies in dogs and cats.