18 Aug 2021
Companion animal anaesthesia: interfascial plane blocks update
Diego Rodrigo Mocholi details the various types of block available and their use in canine, feline and equine veterinary medicine.

An interfascial plane (IP) block consists of the injection of a solution in a fascial plane, from which it spreads within a potential space to affect more extended areas of innervation targets.
The benefit of IP compared to peripheral nerve blocks is that more neural surface may be covered due to the contiguous connection of fascial planes around the body. Also, the IP block techniques tend to be easily and rapidly performed, with higher safety and reduced cost when compared to other locoregional anaesthesia and analgesic therapies.
Erector spinae plane block
The erector spinae muscle group is formed by the spinalis (medial), longissimus (middle) and iliocostalis (lateral) columns (Figure 1).
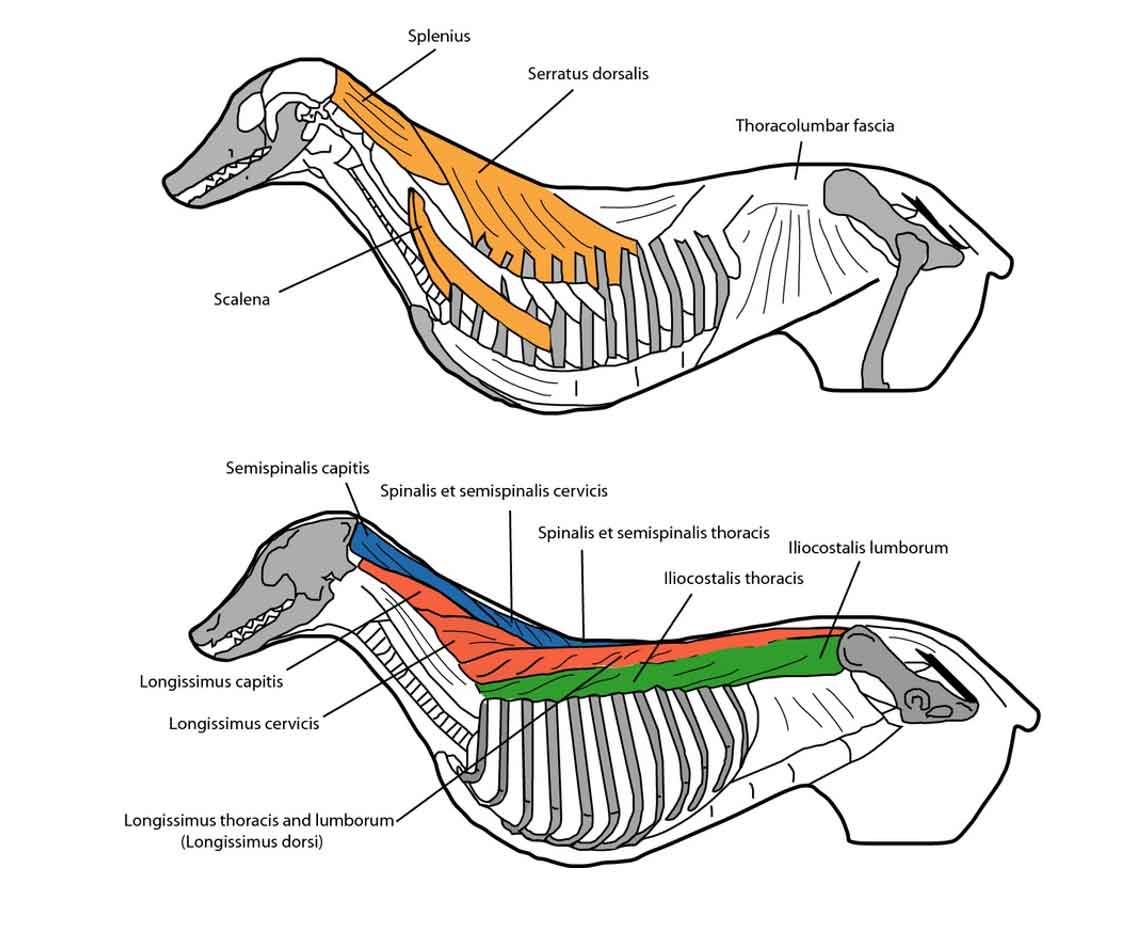
In human medicine, some authors include a fourth group of muscles, the transversospinalis column (which encompasses the semispinalis, multifidus and rotatores muscles), as the more medial area of the erector spinae muscle group. Also, the thoracolumbar fascia covers the erector spinae muscular complex.
The main purpose of the erector spinae plane (ESP) block is the diffusion of the local anaesthesia to the thoracic/lumbar paravertebral space. In veterinary medicine, the thoracic paravertebral space (TPVS) is located on both sides of the vertebral column. The boundaries of the TPVS are formed medially by the bodies of the vertebrae, intervertebral discs and the intervertebral foramen; laterally by the parietal pleura; and the transverse processes and the costotransverse ligaments form the ventral boundary.
The intercostal (spinal) nerves, the dorsal ramus, rami communicantes and the sympathetic chain, and the intercostal vessels lie in the adipose tissue contained in the TPVS. The spinal nerves are segmented into small bundles and lie freely in the adipose tissue of the TPVS, which make them accessible to local anaesthetic solutions injected in the TPVS.
Also, the TPVS communicates with the epidural space medially and with the intercostal space laterally. Although medial boundary is the same for the lumbar paravertebral space (LPVS), the psoas muscle and the transverse processes with their ligaments are the lateral and ventral boundaries, respectively.
The ventral rami of the lumbar spinal nerve roots emerge into the psoas muscle to form the lumbar plexus. Also, the LPVS is communicating medially with the epidural space.
Nowadays, the mechanism of action of the ESP block still remains with some controversy. Human literature has shown certain variability in the spreading of the local anaesthesia. While dorsal ramus spread and minimal to the paravertebral space is the main trend, few studies have shown paravertebral space spreading when either normal or higher volumes are used.
Therefore, the diffusion of local anaesthetics seems mainly in the dorsal ramus, but ventral ramus involvement is found when spreading into either the paravertebral or epidural space.
ESP block in veterinary medicine
The thoracic ESP block has been recently described in canine cadavers. Ferreira et al (2019) performed the ultrasound-guided thoracic ESP block in eight dog cadavers. Both the transverse process (TP) of the thoracic fifth vertebrae and the caudal border of the scapula were used as landmarks.
Dogs were positioned in sternal recumbency, and the linear transducer was sagittally positioned lateral to the dorsal midline and the caudal border of the scapula aligned at the middle of the transducer. The transducer was slightly tilted from medial to lateral until the TP of the fourth, fifth and sixth thoracic vertebrae were visualised.
Then, an insulated needle was caudocranially inserted in an in-plane technique until the tip of the needle was in contact with the dorsal aspect of the TP of the fifth thoracic vertebrae. A total volume of either 0.5ml kg-1 or 1ml kg-1 of methylene blue was injected.
Authors showed no differences in the dorsal rami between both volumes, but higher volume resulted in more extensive muscle spreading. Ventral ramus staining was observed in one out of eight injections when higher volume was used.
Portela et al (2020) described a similar technique for ESP block performed at the TP of the ninth thoracic vertebrae. A linear ultrasound transducer was mediolaterally glided until the image of the TP of the ninth thoracic vertebrae was obtained.
Authors recognised the change in the shape of the bony landmark from the rib (rounded) to the TP (irregular, resembling an armchair; Figure 2).
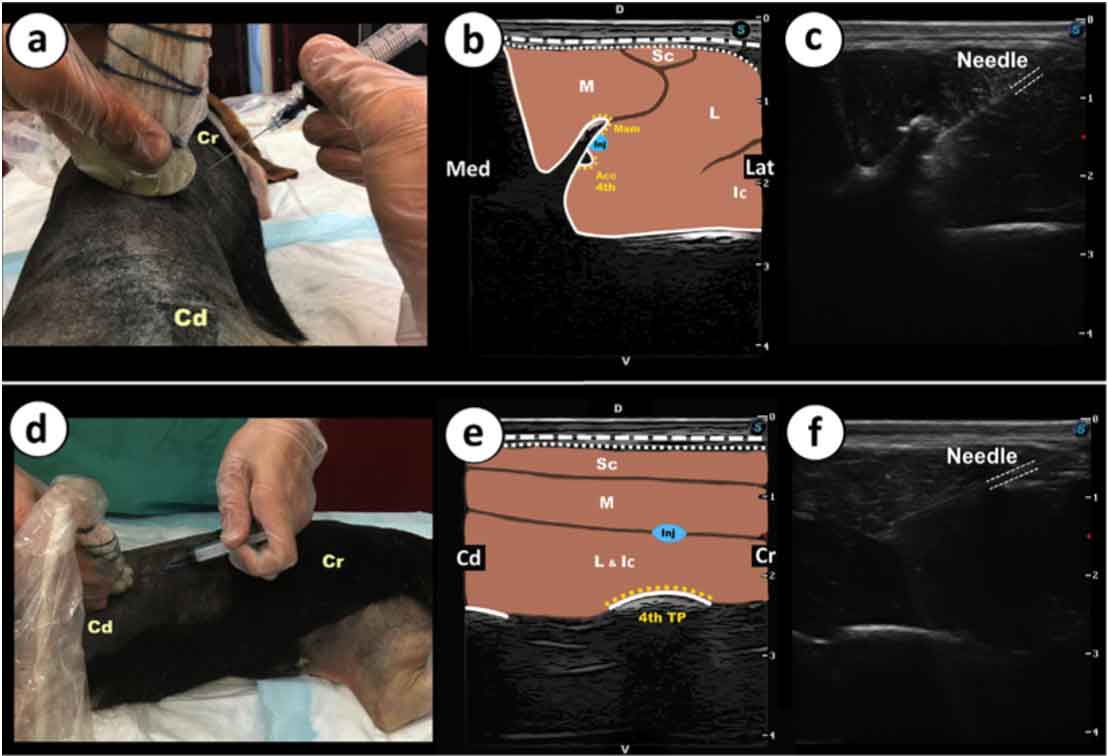
A needle was craniocaudally inserted and visualised in an in-plane technique until the tip of the needle was in contact with the dorsolateral aspect of the TP of the ninth thoracic vertebrae. Low volume (0.3ml kg-1) and high volume (0.6mg kg-1) of a lidocaine-dye solution (2.5ml of a yellow permanent tissue dye was diluted in 250ml lidocaine 2%) were used.
The solution spread medially between the longissimus thoracis and multifidus muscles (medial branches of the dorsal ramus), and laterally between the longissimus thoracis and levatores costarum muscles, and between the longissimus thoracis and iliocostalis thoracis muscles (lateral branches of the dorsal ramus).
Although two cases with the high volume showed some distribution of the solution in the ninth external intercostal muscle, no distribution into the TPVS, ventral rami or intercostal nerve was observed.
Recently, a lumbar ESP block technique has also been studied in canine cadavers. Medina-Serra et al (2021) described two approaches (transversal and parasagittal) to perform an ultrasound-guided ESP block at the level of the fourth lumbar vertebrae.
The transversal approach (TA) was performed by placing a linear transducer perpendicular to the spinous process of the fourth lumbar vertebrae. The transducer was moved until both the accessory process of the fourth lumbar vertebra and the mammillary processes of the fifth lumbar vertebra were visualised.
A needle was inserted in an in-plane technique until the tip contacted the lateral aspect of the mammillary process above the accessory process of the fourth lumbar vertebra. The parasagittal approach (PSA) was performed by placing the spinous process of the fourth lumbar vertebrae in the middle of the transducer, and gliding from medial to lateral until the TP of the fourth lumbar vertebrae was observed.
A needle was craniocaudally inserted until the tip was localised between the multifidi and longissimus lumborum muscles. A total volume of 0.4ml kg-1 was injected for both approaches. A higher degree of dorsal ramus staining was observed with the TA compared to the PSA. An epidural migration was observed when the TA was performed, but no migration occurred during the PSA technique.
More recently, a caudal thoracic ESP block has also been described in equine cadavers. Delgado et al (2021) placed a convex transducer lateral to the dorsal midline at the level of the sixteenth thoracic vertebrae. The transducer was positioned parasagittally and orientated longitudinally just lateral to the dorsal midline once the transverse process of Th16 was identified.
Then, a spinal needle was advanced in a cranial-to-caudal direction and in an in-plane technique until the tip of the needle was at the dorsal surface of the transverse process.
A small volume (5ml to 20ml) of saline solution was injected (the aim was to separate the ESP muscles from the transverse process) prior to dye-lidocaine mixture solution (0.2ml kg-1 of 50:1 ratio of 2% lidocaine and a yellow permanent tissue marking dye). The dorsal branches were completely stained, with minimal diffusion through the vertebral branches (3/20) and the epidural space (20%).
Recent literature has retrospectively evaluated the clinical effects of the thoracic and lumbar ESP block during hemilaminectomy procedures in dogs (Herron et al, 2019; Zannin et al, 2020; Portela et al, 2021). Performing an ESP block has shown potential benefits to reduce perioperative analgesic (opioids and adjuvants) consumption and the likelihood of pharmacological interventions therapy due to cardiovascular adverse events in dogs undergoing hemilaminectomy.
Quadratus lumborum block
The quadratus lumborum (QL) muscle is a hypaxial muscle located ventral to the spinal column that originates from the iliac crest and inserts into the body of the last thoracic vertebrae and the transverse processes of the lumbar vertebrae. Similar to the erector spinae muscle group, the QL muscle is surrounded by the thoracolumbar fascia, a fibrous composite of aponeurotic and fascial tissue.
Based on cadaveric canine studies (Garbin et al, 2020a), the ventral rami of the last two thoracic spinal nerves travel through the bundles of the QL muscle. However, the first lumbar spinal nerves traverse in-between the QL and the psoas, and then through the transversalis fascia and the aponeurosis of insertion of the transversus abdominis muscle.
In human literature, a few possibilities exist for the QL block mechanism of action:
- A spreading to the thoracic paravertebral space exists after the administration of the local anaesthetics between the QL and the transversalis fascia, and along the endothoracic fascia to desensitise the somatic and the thoracic sympathetic trunk.
- A few studies have shown some spreading to the roots and branches of the lumbar plexus.
- Visceral analgesia due to changes in the vasomotor tone of the abdominal branches of the lumbar arteries after blocking the innervation at the thoracolumbar fascia, which induce changes in both the general autonomic tone and local circulation.
QL block in veterinary medicine
Twelve canine cadavers were used to describe an ultrasound-guided QL block technique (Garbin et al, 2020a). Once the dog was positioned in lateral recumbency, an ultrasound transducer was placed parallel and caudal to the last rib until the TP of the first lumbar vertebra and both the QL and the psoas muscles were visualised.
Then, a needle was advanced in an in-plane technique until the tip of the needle was placed in-between the QL and the psoas muscle. A total of eight cadavers were injected with low volume (0.15ml kg-1) and high volume (0.3ml kg-1) of either a coloured lidocaine solution (6/8) or coloured contrast-dye lidocaine solution (2/8). Both solutions were longitudinally spread between the QL muscle and the psoas. More extended areas of spreading (ventral and dorsomedial aspect of the psoas minor muscle, retroperitoneal space dorsal and cranial to the kidneys) were observed when higher volumes were used.
Lately, the same authors described a comparison between two different approaches to perform a lumbar QL block:
- transversal (QLT): same as previously described
- longitudinal (QLL) approach: the transducer is positioned caudal and perpendicular to the last rib (Garbin et al, 2020b)
An in-plane technique was used to advance the needle until the tip was located at the interfacial plane for both approaches. A total of 0.3ml kg-1 of a solution (a mixture containing a tissue marking dye and lidocaine 2%). The results showed a more consistent staining of both sympathetic trunk and spinal nerves when the ultrasound-guided QLT approach was used compared with the QLL technique.
Only one case has reported the use of a transverse approach for an ultrasound-guided QL block with 0.3ml kg-1 ropivacaine in a cat undergoing a cystotomy and explorative laparotomy (Argus et al, 2020). Although a minor amount of perioperative analgesia was used, further research is needed in cats.
Serratus plane block
In veterinary medicine, the serratus ventralis thoracis muscle lies beneath the cutaneous trunci and latissimus dorsi muscles in dogs. The serratus ventralis thoracis muscle originates cranially to the first rib and extends rostrally as the serratus ventralis cervicis, and ventrally to the scalenous and external oblique abdominal muscles.
The serratus ventralis thoracis muscle is caudally inserted up to the seventh rib. The long thoracic nerve runs horizontally through the lateral surface of the muscle, while lateral cutaneous branches of thoracic nerves (T4 to T7) and the intercostobrachial III nerve are located at the ventral edge. Both the long thoracic nerve and group of lateral cutaneous branches are responsible for the mechanism action of the serratus plane (SP) block.
SP block in veterinary medicine
A canine cadaveric study described the superficial SP block technique (Freitag et al, 2019). A linear ultrasound transducer was placed at the level of the shoulder joint, and perpendicular to the fourth and fifth rib. Then, the needle was caudocranially introduced in an in-plane technique until the tip of the needle was aimed to the interfascial plane between the latissimus dorsi and the serratus ventralis thoracis muscles.
Three different volumes (0.3ml kg-1, 0.6ml kg-1 and 1ml kg-1) of a ropivacaine-methylene blue solution (1:1) were injected. No differences existed among the three groups about the spread of solution and dermatomal area covering. Long thoracic nerve staining rate was higher (88.5%) compared to intercostobrachial nerves (30%).
Drożdżyńska et al (2017a) described the deep SP block technique. The dye-contrast solution was administered in the fascial plane between the serratus ventralis thoracis and external intercostal muscles at two different injection points:
- a volume of 0.5ml kg-1 at the fourth intercostal space and below the ventral border of the scapula
- 0.5ml kg-1 at the level of the fifth intercostal space. Staining was covered between the first and the sixth intercostal space
Teixeira et al (2017) reported a case series with four female dogs undergoing mastectomy. The superficial SP block with bupivacaine 0.25% (0.3ml kg-1) may be a complementary technique when combined with the transversus abdominis plane (TAP) block to manage preoperative analgesia in bitches undergoing mastectomy.
Another case series article described the positive benefits (prevent nociception and reduce intraoperative opioid requirements) of the deep SP block technique in dogs undergoing thoracotomy.
TAP block
The transversus abdominis muscle is one of the hypaxial muscle layers, which, together with both the external and internal abdominal oblique muscles, conform the lateral abdominal wall. Sensory afferent nerve branches of the last thoracic and first lumbar vertebrae run through the fascial plane between the transverses abdominis and internal abdominal oblique muscle, and are the therapeutic target when local analgesia is administered by the TAP block technique.
TAP block in canine species
The first description of the TAP block in canine cadavers was made by Schroeder et al (2011). The linear transducer was positioned perpendicular to the dorsal midline, and between the caudal aspect of the last rib and the iliac crest.
Once the three layers of the abdominal wall (external and internal abdominal oblique, and transversus abdominis muscles) were visualised, a Tuohy needle was inserted in an in-plane technique until the needle tip reached the interfacial plane between the internal abdominal oblique and the transversus abdominis muscles. A total volume of 10ml of a 1:1 mixture solution (methylene blue and 0.25% bupivacaine) was injected.
Results of the study showed higher success rates of staining were found from T12 to L2 nerves (80% to 100%) compared to T11 and L3 (20% and 30%, respectively).
Although the aforementioned study showed promising results, a recent study found some anatomical variations in the thoracolumbar nerve branches in dogs (Castañeda-Herrera et al, 2017).
Authors described that branches from T7 to T9 are also involved in the innervation of the cranial abdominal wall. Therefore, further research has been focused on new techniques, approaches or increasing the solution volumes that would improve nerve staining in more cranial and caudal areas of the abdomen:
Volume
A few studies have shown that lower volumes (either 0.3ml kg-1 or 0.5ml kg-1) showed similar staining with no significant differences compared to higher volumes (0.6ml kg-1 to 1ml kg-1; Zoff et al, 2017; Freitag et al, 2021a).
Solution
De Miguel Garcia et al (2020) compared the spreading of three different solutions:
- 1% methylene blue
- a 50:50 mixture of 1% methylene blue and 0.5% bupivacaine
- a 25:75 mixture of 1% methylene blue and iohexol as contrast agent
Although the same volume was injected (0.5ml kg-1), a significantly more dorsoventral spreading was found with the solution (1) compared to the mixture of methylene blue and iohexol.
Two-point injections
Johnson et al (2018) described two different points to perform the TAP block:
Cranial: the transducer is positioned transversal to the dorsal midline and caudal to the thirteenth rib.
Caudal: the transducer is placed cranial to the tuber coxae. A total volume of 0.3ml kg-1 per site of injection was administered. Results showed that only the branches from T13 to L3 were successfully stained.
Different approach
A subcostal oblique TAP block has been described in canine cadavers (Drożdżyńska et al, 2017b). First, the transducer was placed transversal to the linea alba and caudal to the diploid process. Then, the transducer was rotated 10° to 15° and parallel to the costal arch until the rectus abdominis and transversus abdominis muscles were visualised.
The needle was advanced using an in-plane technique until the tip was placed in-between the rectus abdominis and transversus abdominis muscles. A total volume of 10ml of 1% methylene blue was divided in three different points: after first injection, the researcher moved the transducer caudolaterally until the point at which the typical fluid “pocket” terminated.
T10, T11 and T12 were the most stained (95% to 100%), T9 showed good results (72%) and T13 had a success staining rate of 61%. More recently, Romano et al (2021) compared the described technique by Johnson et al (2018) to a new two-point injection (SL approach): a single-point modified from Drożdżyńska et al (2017b) plus a single-point with the transducer perpendicular to the midline and cranial to the umbilucus.
A total volume of 1ml kg-1 of a mixture (1:9) of 0.39% methylene blue and sodium chloride was divided in the four different points. The SL approach showed a higher success rate of staining (80.3%) compared to the Johnson et al technique (53.5%).
The TAP block has shown good results in dogs undergoing mastectomy (Portela et al, 2014; Teixeira et al, 2017). Better perioperative pain management has been recorded when TAP block in combination with other nerve blocks (intercostal or serratus plane nerve blocks) were used in the protocol during mastectomy procedures in bitches.
Recently, a continuous bilateral TAP block has been used for pain management in two dogs with severe pancreatitis and a third one undergoing an explorative laparotomy (Freitag et al, 2018). A dose of 0.3ml kg-1 of 0.5% bupivacaine was administered every six hours through the catheter placed in the fascia between the internal abdominis oblique and transversus abdominis muscles. Pain scoring was reduced during local anaesthetic therapy, and no adverse events were recorded.
TAP block in feline species
Eight cat cadavers have been used to describe the anatomy and to perform a comparison of two different techniques for TAP block in cats (Otero et al, 2021). The ultrasound-guided TAP injections were performed as follows:
- Two-point injection (LL): the transducer was positioned in the midline and cranial to the umbilicus. Then, the probe was laterally moved until the internal abdominis oblique and transversus abdominis muscles were identified. The cranial point of injection was caudal to the last ribs, and the caudal point was cranial to the iliac crest.
- Three-point technique (SL): an extra point of injection was added to the previous technique. The transducer was located parallel to the costal arch.
A total volume of 0.5ml kg-1 of a mixture (1:1) of 1% methylene blue and 2% lidocaine was divided in aliquots for each point of injection. A higher success rate of staining was recorded for the SL approach compared to the LL technique (92.6% and 66.7%, respectively).
A previous study already showed the clinical effect of the TAP block in cats undergoing ovariectomy (Skouropoulou et al, 2018). Authors performed the TAP block by placing the linear transducer 3cm to 4cm parallel to the ventral midline, and caudal to the last rib and cranial to the iliac crest.
Twenty cats were recruited for the study and divided in two groups based on drug administration:
- BL group: 0.2ml kg-1 of 0.5% bupivacaine completed with 2% lidocaine up to a total volume of 1.5ml
- C group: 1.5ml of sodium chloride
Total pain score was significantly higher in the C group during the 24 hours after extubation. Perioperative rescue analgesia was required in the C group. The BL group showed a low pain score (below three) at each time point.
TAP block in equine species
The 10 intercostal nerves (T9 to T17), T18, and L1 and L2 are responsible for the innervation of the abdominal wall in horses.
Baldo et al (2018) were the first to describe an ultrasound-guided technique for TAP block in the equine species. Four pony cadavers were used in this study. The linear transducer was positioned in a midpoint between the most caudal aspect of the last rib and the most cranial aspect of the iliac crest, and caudal to the umbilicus.
A total volume of 0.5mg kg-1 of a 1:1 solution (1% methylene blue and 0.5% bupivacaine) was injected. T16 showed the higher success rate of staining (100%), followed by T17, T18 and L1 (87.5%), and T15 (75%). Then, authors recommended further studies about more cranial techniques for TAP blocks.
Lately, Küls et al (2020) described a three-point injection technique in ponies. The landmarks for this modified technique were as follows:
- T18: similar as previously described in Baldo et al (2018)
- T14: 10cm ventral to the fourteenth costochondral joint
- T8: caudolateral to he xiophoid process and 10cm ventral to the T9 costochondral joint
Total volume per hemiabdomen was 0.3ml kg-1 of a mixture (1:1) of methylene blue and 0.5% bupivacaine, and divided in aliquots per each point of injection. Results showed a length of staining more than 1cm in nerves from T8 to T18. Also, this modified TAP block technique showed a dermatomal desensitisation of the ventral abdomen up to two hours.
Recently, a modified subcostal approach for the TAP block has been described in horse cadavers (Freitag et al, 2021b). Three anatomical landmarks are important for this approach: the xiphoid cartilage, umbilicus and the cutaneous trunci muscle. Two injection points are located to perform this technique:
- midpoint between the xiphoid cartilage and the umbilicus
- the second point is located in the third part of an imaginary three portions division of the area between the first point and the end caudal half of the abdomen
A total volume between 0.12ml kg-1 and 0.16ml kg-1 of a dye solution was used. Lateral recumbency position showed a higher number of nerve staining (from T9 to T17) compared to horses in dorsal recumbency (T13 to T18).
