1 Jul 2025
Corneal ulcer update – case management and new therapies
Chris Dixon BVSc, CertVOphthal, MRCVS covers the different forms of this common ocular issue and the emerging surgical and treatment options for them.

Figure 1. The corneal layers in an anatomical diagram.
Corneal ulcers are among the most common ocular emergencies in veterinary practice. They result from a breach in the cornea’s protective epithelium and can lead to pain, vision impairment and a loss of sight.
Ulcers can range from simple superficial abrasions that heal quickly to deep, melting or perforating lesions that threaten the eye’s integrity. We have seen significant advances in our understanding and treatment of corneal ulceration in human and veterinary patients, including novel therapies to combat infection and strengthen the cornea.
This update will review current best practices for diagnosis and classification of corneal ulcers, standard treatments for superficial ulceration, and the management of complicated ulcers (deep, infected, malacic, descemetoceles and perforations).
The author will also highlight the latest research, from cross-linking techniques to emerging modalities such as ultraviolet-C (UVC) therapy and novel compounds under investigation.
Anatomy of the cornea
The cornea is a transparent, avascular structure forming the anterior wall of the eye. The cornea is the principal refractive structure of the eye, and in dogs, the cornea is approximately 0.5mm to 0.6mm thick, with the central region being slightly thinner than the periphery (Gilger et al, 1991).
The cornea’s unique anatomy contributes to maintaining corneal transparency and optical function, and consists of three primary layers:
Corneal epithelium: comprising non-keratinised, stratified, squamous epithelial cells that undergo constant shedding and basal cell proliferation. The epithelium forms about 10% of the corneal thickness and it is attached to the underlying stroma layer.
Corneal stroma: this layer constitutes 90% of the corneal thickness and consists of collagen fibres arranged in parallel layers with interspersed keratocytes. The corneal stroma provides mechanical strength.
Corneal endothelium: the innermost layer, consisting of a single layer of cells, is responsible for dehydrating the cornea. The endothelium’s basement membrane is known as Descemet’s membrane.
Diagnosis and classification of corneal ulcers
Accurate diagnosis and classification of corneal ulcers is critical to effective management.
Any patient presenting with a painful eye should be assessed for corneal ulceration, and particular attention should be focused on any potential source of mechanical abrasion (such as trauma, entropion and distichiasis).
If an ocular discharge is present, sampling for cytology and laboratory submission for the identification of pathogens (such as bacterial, fungal or viral) should be considered.
Superficial ulcers involve only the epithelium and exposed anterior stroma, whereas deeper ulcers extend into mid to deep stroma, and a descemetocele is an ulcer reaching Descemet’s membrane (appearing as a protruding, central clear window within the ulcer bed). A perforation indicates full-thickness rupture with aqueous humour leakage and potential iris prolapse.
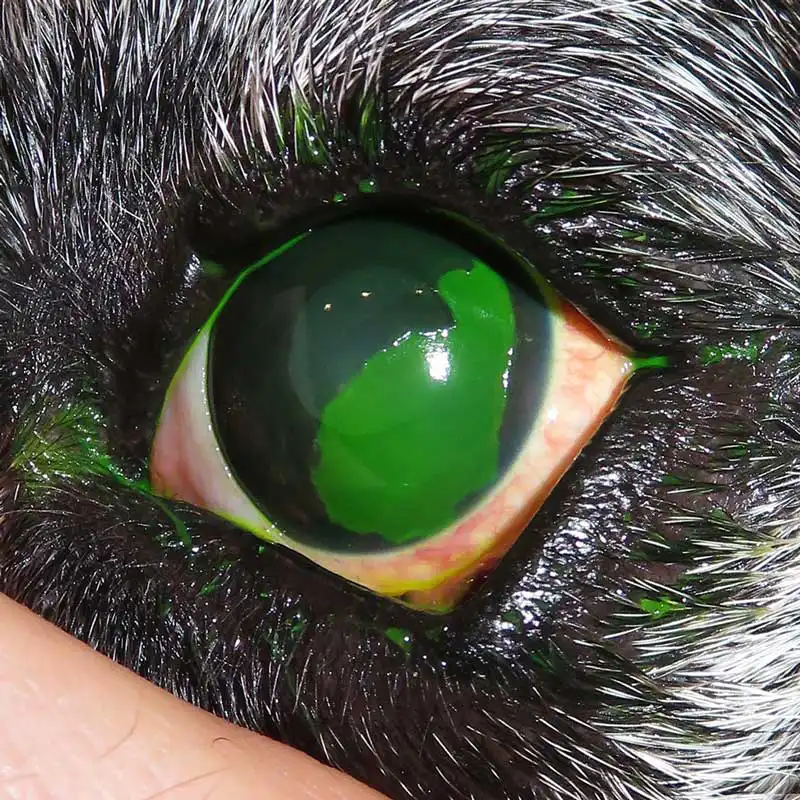
Infected ulcers are recognised by stromal loss, malacia (a gelatinous melting appearance) and often corneal infiltrates or discharge.
Early diagnostic tests should include corneal cytology and culture to identify pathogens and guide appropriate therapy. In practice, while awaiting culture results, broad-spectrum topical antimicrobials are started empirically, along with anti-collagenase therapy if melting is present, and atropine for pain (reflex uveitis).
It is critical to assess ulcer depth with magnification, with ulcers involving more than or equal to 50% stromal depth carrying a high risk of progression or perforation, and they often warrant surgical intervention.
A Seidel test (fluorescein diluted by aqueous humour) can detect micro-perforation or leakage.
Species and breed differences influence both predisposition and diagnostic approach. Brachycephalic dogs are prone to corneal ulcers due to prominent globes and inadequate blinking. In cats, feline herpesvirus-1 is a common cause of corneal ulceration (classically dendritic pattern), and chronic cases may lead to the development of corneal sequestrae. Horses have a higher incidence of fungal ulcerative keratitis compared to other domesticated species, and rabbits commonly develop ulcers secondary to environmental trauma and dental disease.
Once an ulcer is confirmed, it should be categorised by factors including shape, size, depth and complexity:
- superficial versus deep
- large versus focal
- axial versus peripheral
- infected versus clean
- melting versus non-melting
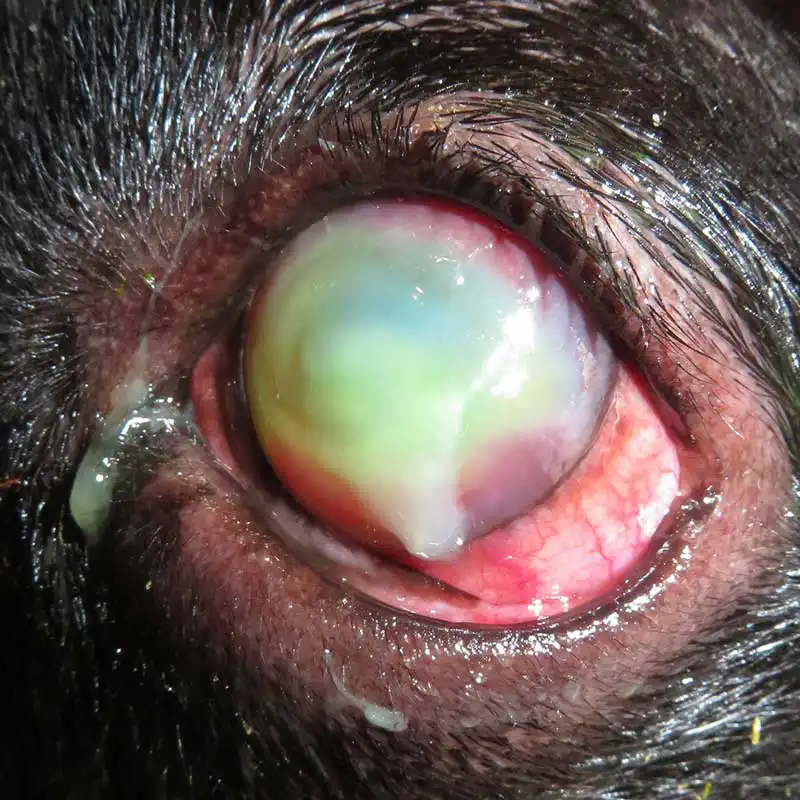
Management of superficial and uncomplicated ulcers
Most superficial corneal ulcers heal rapidly with appropriate supportive medical therapy.
The cornerstones of treatment are broad-spectrum topical antibiotics to prevent opportunistic infection, topical cycloplegics (such as atropine) to relieve ciliary body spasm, topical lubrication to limit abrasion of the epithelial cells and systemic analgesia for discomfort. The selection of antibiotic should be dictated by the type of infection and its likely susceptibility to an antibiotic class while following the prescribing cascade.
The most common organisms found in infected corneal ulcers are Gram-positive cocci (Staphylococcus species or Streptococcus species) or Gram-negative rods (Pseudomonas species). In-house cytology can determine the type of bacteria present before bacteriology results are processed by a laboratory, and provide a headstart when considering an appropriate antibiotic.
Topical corticosteroids are contraindicated for the management of corneal ulceration, as they may impede healing and promote infection and collagenolysis. Owners should be instructed to use a protective Elizabethan collar or ocular shield to prevent self-trauma during the healing process. Topical local anaesthetics (such as proxymetacaine and tetracaine) should not be dispensed due to their epitheliotoxicity and may significantly delay healing or allow for severe secondary complications. The placement of a soft bandage lens can provide mechanical protection to the cornea, and research has shown that they may increase comfort and reduce the time taken for a corneal ulcer to heal.
Uncomplicated superficial ulcers will usually heal within three to seven days, with epithelial cells migrating from the limbus in a centripetal pattern. Cenedella and Fleschner (1990) reported that complete replacement of the corneal epithelium can occur within two weeks.
Spontaneous chronic corneal epithelial defects
If a superficial ulcer fails to heal within an expected time frame (21 days), the cornea should be evaluated for the possibility of a spontaneous chronic corneal epithelial defect (SCCED). This condition is most common in dogs older then seven years and occurs as a result of a failed attachment between the epithelium and stroma.
Research continues into the exact mechanism of the pathophysiology, and a recent paper by Meurs et al (2021) has identified a gene mutation (NOG) in the boxer dog that may explain a predisposition to SCCED development in this breed.
To facilitate corneal healing, the loose epithelium must be removed and the abnormal basement membrane destroyed. Published studies demonstrate 80% to 90% healing with a diamond burr or grid keratotomy in canine patients. This should not be performed in feline patients due to the high likelihood of secondary sequestrae formation. Stanley et al (1998) and Peiffer et al (1976) reported increased success when managing SCCED cases with a superficial keratectomy, but this procedure requires general anaesthesia in small animal patients, and access to specialised microsurgical instruments and training.
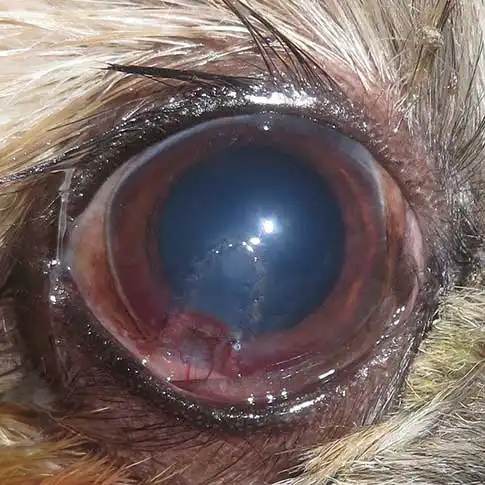
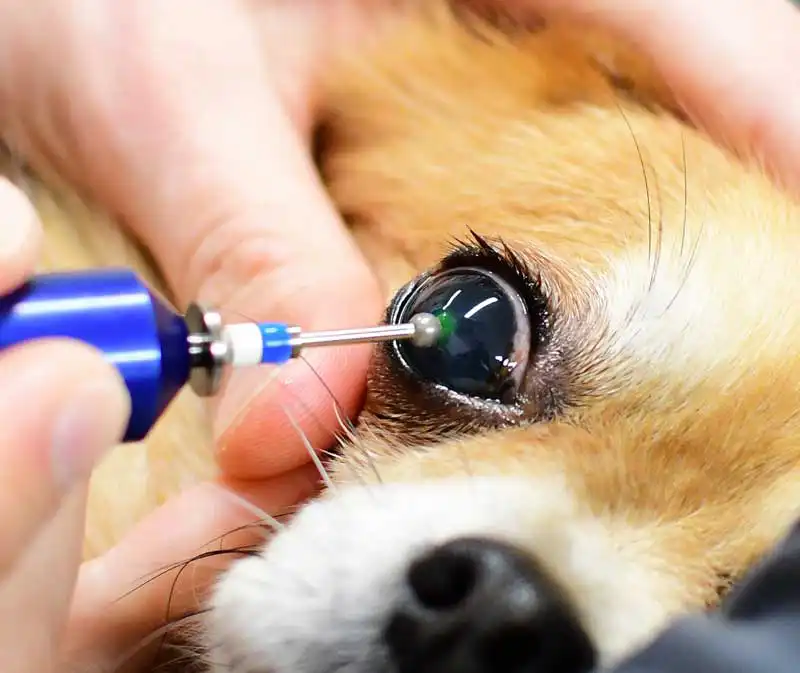
Management of deep, infected and complicated ulcers
When an ulcer is deep or infected, more aggressive therapy and sometimes surgical intervention are required. Key principles are to halt corneal destruction, eradicate infection and support corneal healing.
Infected ulcers usually involve bacterial colonisation, but consideration must be made for other pathogens, which may include viral, fungal or algal organisms. Infected corneal tissue can appear off-white or yellow, and may have associated oedema and vascularisation within the surrounding tissue. Keratomalacia (melting ulcer) is the degeneration and liquefaction of the stroma caused by proteolytic enzymes leading to a rapid loss of normal corneal architecture.
Intensive, frequent topical antimicrobial therapy is indicated: typically, a broad-spectrum bactericidal antibiotic is applied every one to two hours, initially. If culture results identify a specific pathogen, therapy can be tailored, and for keratomalacia, anti-collagenase/protease treatments should be started immediately to prevent rapid stromal dissolution. These include topical autologous serum, plasma, ethylenediaminetetraacetic acid, N-acetylcysteine and tetracycline antibiotics that have anti-collagenase activity.
If an ulcer is “deep” (with more than half stromal thickness loss) or progressing despite medical therapy, surgical intervention is often required to stabilise the tissue. Early diagnosis and prompt treatment is critical to successful management of these cases – especially when considering the possibility of active infection within the cornea.
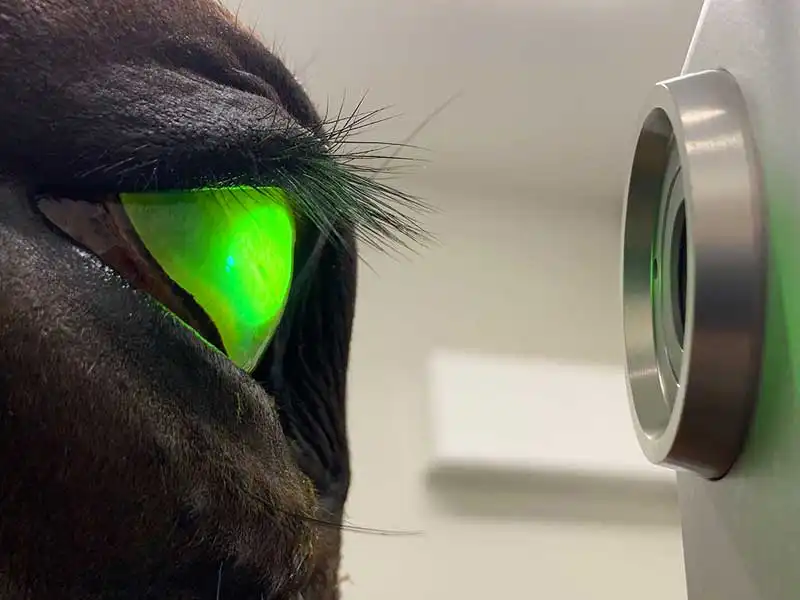
Descemet’s membrane is transparent, extremely thin (3µm to 12µm) and fragile. A descemetocele is the deepest possible corneal ulcer before perforation occurs and it is a surgical emergency.
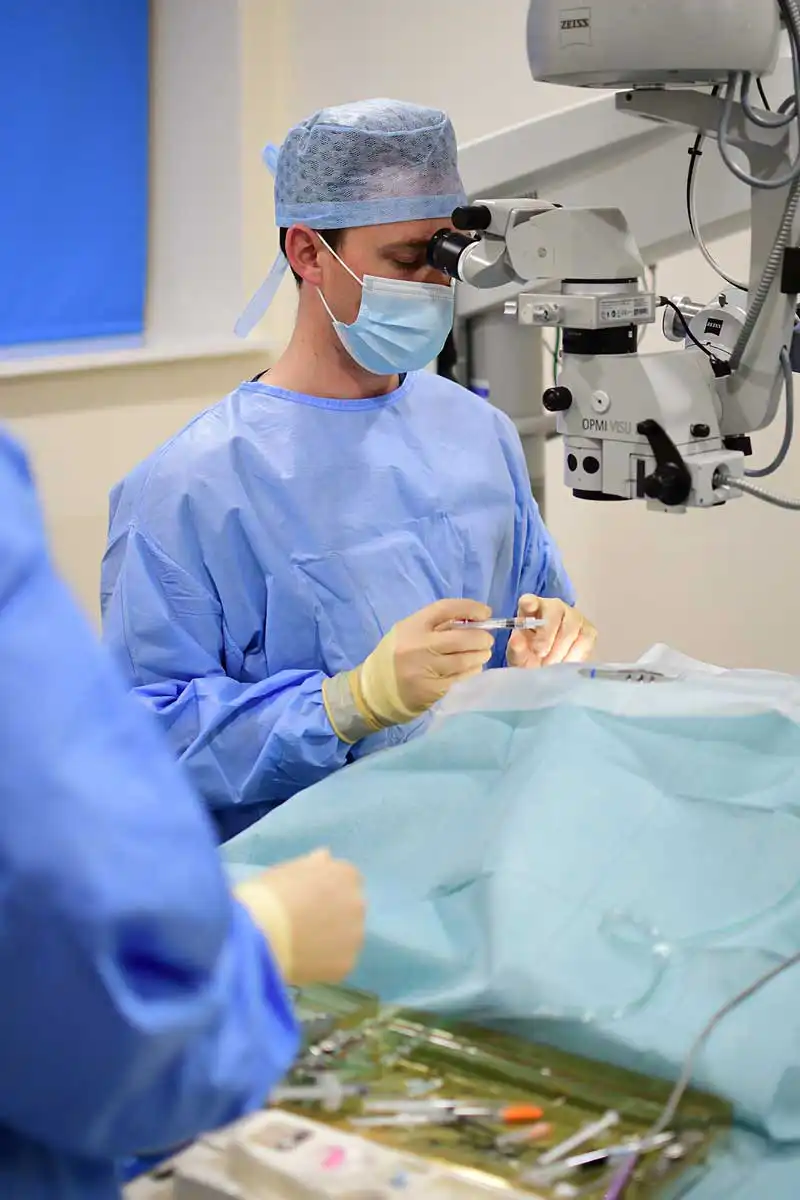
Performing corneal surgery necessitates microsurgical expertise and specialised instruments, with the choice of graft technique depending on the ulcer’s size and location. Ophthalmologists frequently use autografts, allografts or xenografts to replace compromised corneal tissue. Ongoing research has led to improvements in surgical methods and materials, and various studies have evaluated the outcomes of different grafting approaches.
Conjunctival grafts (pedicle or bridge flaps) can be employed to provide blood supply and protection to exposed stroma, but do not deliver the structural support of transposed corneal tissue or xenograft biomaterials.
Corneoconjunctival transposition is a type of sliding lamellar keratoplasty commonly employed by veterinary ophthalmologists to manage deep corneal ulcers. It involves shifting a segment of healthy adjacent corneal tissue, along with the attached limbus and conjunctiva, and securing it with fine sutures (9/0 to 11/0). Including the limbus and conjunctiva enhances graft viability and shortens the distance for cellular migration.
Xenografts
Xenograft and bioengineered materials represent significant advancements in the surgical management of complex, deep corneal ulcers – particularly where autologous graft tissues are limited or unavailable.
Recent developments have expanded available options to include novel xenograft materials:
- Amniotic membranes (porcine, equine, bovine or human).
- Porcine small intestine submucosa.
- Porcine urinary bladder submucosa.
- Bio-engineered, porcine collagen xenograft.
- Bovine pericardium.
These materials offer valuable alternatives in challenging clinical scenarios, allowing preservation of globe integrity in cases previously considered unsalvageable. However, we must consider the likely trade off between globe preservation and the potential compromise in long-term visual clarity, as significant corneal opacification may occur postoperatively.
Further research and clinical follow-up studies continue to refine indications and improve outcomes for these innovative treatments.
Advanced and emerging therapies for complicated ulcers
Advancements in veterinary ophthalmology are providing new tools to manage complicated corneal ulcers – especially those that are infected or melting.
Traditional therapy has relied on antimicrobials and surgery, but newer approaches aim to strengthen the cornea and eliminate infection in novel ways.
Corneal collagen cross-linking
Corneal cross-linking (CXL) is a minimally invasive therapeutic procedure originally developed in human ophthalmology for managing keratoconus, corneal ectasia and other degenerative corneal diseases. The fundamental principle involves strengthening the corneal stroma by creating additional chemical bonds between collagen fibres through ultraviolet-A (UVA) irradiation combined with riboflavin (vitamin B2).
In veterinary ophthalmology, keratomalacia and microbial corneal disease present significant therapeutic challenges especially when considering rising antibiotic resistance. Corneal cross-linking has demonstrated promising results in managing these conditions. Studies on CXL in veterinary medicine predominantly focus on canine, feline and equine populations.
Numerous papers have been published over the past decade, demonstrating the potential benefit of CXL as an adjunctive treatment choice. Reports have indicated that photo-activated chromophore for keratitis-CXL (PACK-CXL) can potentially create a substantial improvement in corneal structural integrity, reduce the risk of treatment failure and improve healing outcomes (Kowalska et al, 2023; Shulka et al, 2025). Marchegiani et al (2022) found riboflavin/UVA corneal phototherapy is effective as standalone management for canine ulcerative keratitis, and Suter et al (2022) emphasised the bactericidal potential of accelerated protocols, potentially broadening treatment efficacy against bacterial keratitis.
UVC therapy
UVC refers to UV light in the 100nm to 280nm range, which has potent germicidal effects. At 265nm (the wavelength often used in devices), UVC is highly absorbed by microbial DNA/RNA, causing pyrimidine dimer formation that prevents pathogen replication and results in cell death.
Crucially, UVC can inactivate bacteria, fungi and even protozoa, and it works independently of antibiotic resistance because microbes cannot easily develop resistance to direct DNA damage. UVC directly kills microbes and does not provide any cross-linking effect, as seen with PACK-CXL.
Published studies have assessed the risk of DNA damage associated with UVC, demonstrating that controlled low-dose exposure did not result in notable genetic mutations or cytotoxicity in corneal epithelial cells. The safety evaluations involved in vitro experiments with human corneal cells, in vivo assessments using mouse corneas and ex vivo studies on porcine corneas.
Recent research has focused on delivering controlled low-dose UVC to the cornea to kill pathogens while minimising host tissue effects. Dean et al (2011) conducted one of the first in vitro vet studies using UVC on agar cultures and found that even a few seconds of UVC exposure could inhibit growth of common bacteria, with no significant death of corneal cells in culture. In a preclinical study by Marasini et al (2022), a low-dose 265nm UVC regimen (less than or equal to five seconds of exposure) was shown to inhibit a broad range of bacteria and fungi, including antibiotic-resistant strains, and it did not cause detectable DNA damage in corneal tissue or deeper structure penetration beyond the superficial stroma. These findings have led to development of the UVC devices for clinical use.

Genipin
Genipin is a naturally occurring compound (derived from the gardenia fruit) that has gained attention as an alternative cross-linking agent. Unlike riboflavin, which requires UVA light, genipin is a small molecule that can chemically cross-link collagen and strengthen tissues.
Ophthalmic researchers have found that genipin-treated corneal tissue becomes significantly more resistant to enzymatic digestion, and genipin appears to have direct antimicrobial effects. In 2025, Slenter et al presented a study titled, “The in vitro antibacterial effects of genipin against pathogens commonly associated with canine infected ulcerative keratitis”, which provided further evidence that genipin can inhibit canine corneal pathogens”. While details of that study await full publication, it aligns with the idea that genipin could serve a dual purpose in ulcer management, strengthening the corneal stroma and eliminating infection.
At present, genipin is not yet available as a clinical treatment for corneal ulcers, and research is ongoing to determine safe dosing and delivery.
Cold atmospheric plasma
Cold atmospheric plasma (CAP) is a partially ionised gas that includes electrons, ions, ultraviolet radiation, electromagnetic fields, and reactive oxygen and nitrogen species.
CAP has been principally used for treating skin wounds and has gained interest because of its antimicrobial properties, which may include efficacy against multidrug-resistant pathogens. CAP is a low-temperature plasma (less than 40°C), and preliminary findings presented by Winter et al (2025) at the European College of Veterinary Ophthalmologists and by Zoeggeler et al (2025) at the International Equine Ophthalmology Consortium conferences suggest that CAP may suitable for direct use on infected equine corneal tissue.
Other novel compounds and methods under exploration include photodynamic therapy (such as rose bengal, toluidine blue and infracyanine green activated by specific wavelengths of light), tissue engineering, regenerative therapies and novel drug delivery systems.
Conclusion
Corneal ulcer management has evolved significantly, yet the foundation remains of prompt diagnosis and appropriate intervention before complications arise.
The priority is to recognise ulcer severity (superficial versus complicated), initiate appropriate broad-spectrum antimicrobial and anti-melting therapy when needed, and know when to refer cases to an ophthalmologist for advanced therapies or surgery.
Continued research in veterinary ophthalmology is enhancing our knowledge and treatment approaches for corneal ulcers. The potential integration of safe, novel therapies with sound clinical principles into our “toolbox” may improve outcomes for patients suffering from even the most complicated corneal ulcers, enabling us to deliver improved patient care.
Use of some of the drugs in this article is under the veterinary medicine cascade.
- Article appeared in Vet Times (2025), Volume 55, Issue 26, Pages 6-10.
References
- Cenedella RJ and Fleschner CR (1990). Kinetics of corneal epithelium turnover in vivo: studies of lovastatin, Investigative Ophthalmology and Visual Science 31(10): 1,957-1,962.
- Dean SJ, Petty A, Swift S, McGhee JJ, Sharma A, Shah S and Craig JP (2011). Efficacy and safety assessment of a novel ultraviolet C device for treating corneal bacterial infections, Clinical and Experimental Ophthalmology 39(2): 156-163.
- Gilger BC, Whitley RD, McLaughlin SA, Wright JC and Drane JW (1991). Canine corneal thickness measured by ultrasonic pachymetry, American Journal of Veterinary Research 52(10): 1,570-1,572.
- Kowalska ME, Shukla AK, Arteaga K, Crasta M, Dixon C, Famose F, Hartnack S and Pot SA (2023). Evaluation of risk factors for treatment failure in canine patients undergoing photoactivated chromophore for keratitis - corneal cross-linking (PACK-CXL): a retrospective study using additive Bayesian network analysis, BMC Veterinary Research 19(1): 227.
- Marasini S, Dean SJ, Swift S, Perera J, Rupenthal ID, Wang T, Read H and Craig JP (2022). Preclinical confirmation of UVC efficacy in treating infectious keratitis, The Ocular Surface 25: 76–86.
- Marchegiani A, Gialletti R, Cassarani MP, Cerquetella M, Attili AR, Lombardo G, Lombardo M, Spaterna A and Arcelli R (2022). Riboflavin/UV-A corneal phototherapy as stand-alone management of ulcerative keratitis in dogs, Veterinární Medicína 67(4): 190-198.
- Meurs KM, Montgomery K, Friedenberg SG, Williams B and Gilger BC (2021). A defect in the NOG gene increases susceptibility to spontaneous superficial chronic corneal epithelial defects (SCCED) in boxer dogs, BMC Veterinary Research 17(1): 254.
- Peiffer RL Jr, Gelatt KN and Gwin RM (1976). Superficial keratectomy in the management of indolent ulcers of the Boxer cornea, Canine Practice 3(4): 31-33.
- Shukla AK, Kowalska ME, Arteaga K, Crasta M, Dixon C, Famose F, Hartnack S and Pot SA (2025). Evaluation of photoactivated chromophore for keratitis-corneal cross-linking (PACK-CXL) in feline infectious keratitis—patient demographics, treatment protocols, risk factors, and treatment outcome: a retrospective study, Veterinary Ophthalmology 28(2): 330-340.
- Slenter IJM, Smit AA, Schipper BW, Broekhuizen-Stins MJ, van der Graaf-van Bloois L, Djajadiningrat-Laanen SC and Broens EM (2025). The in vitro antibacterial effects of genipin against pathogens commonly associated with canine infected ulcerative keratitis: a preliminary study, Proceedings of the European College of Veterinary Ophthalmologists (ECVO) Annual Meeting 2025, Edinburgh, abstract #143.
- Stanley RG, Hardman C and Johnson BW (1998). Results of grid keratotomy, superficial keratectomy and debridement for the management of persistent corneal erosions in 92 dogs, Veterinary Ophthalmology 1(4): 233-238.
- Suter A, Schmitt S, Hübschke E, Kowalska M, Hartnack S and Pot SA (2022). The bactericidal effect of two photoactivated chromophore for keratitis-corneal crosslinking protocols (standard vs. accelerated) on bacterial isolates associated with infectious keratitis in companion animals, BMC Veterinary Research 18(1): 317.
- Winter N, Oltmanns H, Meißner J, Marx A, Heun F, Schieder A-K, Volk H, Ohnesorge B and Busse C (2025). Argon cold atmospheric plasma eradicates in vitro pathogens associated with equine infectious keratitis, Proceedings of the European College of Veterinary Ophthalmologists (ECVO) Annual Meeting 2025, Edinburgh, abstract #102.
- Zoeggeler A, Jung A, Soukup P, Koller M and Stadler S (2025). Use of argon cold plasma treatment in horses with fungal and bacterial infections: a case series, Proceedings of the International Equine Ophthalmology Consortium (IEOC) Annual Meeting 2025, Lucerne, abstract #10.
