7 May 2024
Anna Lena Kraemer DipACVIM, MRCVS and Ian Ramsey BVSc, PhD, DipECVIM-CA, DSAM FHEA, FRCVS provide a widespread overview of new medications, including when and where to use them, plus look at glucose monitoring.

Image © julijadmi / Adobe Stock
Diabetes mellitus (DM) represents one of the most common endocrine disorders in cats and dogs.
Most cats suffer from the type of diabetes mimicking type 2 DM in humans, which is characterised by insulin resistance and relative insulin deficiency, in contrast to dogs, which most often suffer from type 1-like DM (absolute insulin deficiency).
A number of exciting new medications and management options for DM have emerged on to the veterinary market of late. In the UK, the launch of our first oral anti-diabetic – a sodium-glucose cotransporter 2 (SGLT2) inhibitor called velagliflozin (Senvelgo; Boehringer Ingelheim) – marks a paradigm shift in feline diabetology.
We are likely to see more SGLT2 inhibitors released on to the UK market soon (bexagliflozin is already sold in the US with the trade name Bexacat; Elanco). Despite several scientific studies – some published; some undertaken as part of licensing requirements – much can still be learned.
This article will give you a comprehensive overview of when and how to use these drugs, the side affects you should anticipate and how to react when you encounter the most feared complication of SGLT2 inhibitors – euglycaemic diabetic ketoacidosis (eDKA).
It will furthermore cover the advantages and disadvantages of continuous glucose monitoring in both cats and dogs – and, lastly, the value of measuring haemoglobin A1c in dogs.
Glucose toxicity refers to the adverse effects of glucose on cell physiology – specifically islet cell physiology.
It has been known for many years that chronic excess of glucose impairs insulin secretion – leading to a vicious circle of rising glucose concentrations and decreased insulin secretion.
Initially, these changes are reversible and the impaired insulin secretion observed in early human type 2 DM can improve significantly with control of the hyperglycaemia. Ultimately, however, the damage to the beta cells becomes irreversible (“beta cell failure”) and, in effect, the patient becomes a type 1 insulin-dependent diabetic. Several reasons may explain why high concentrations of glucose may reduce islet cell function and these processes may evolve as the DM progresses.
Oxidative stress, increased free fatty acids, increased peripheral resistance and non-enzymatic glycosylation of proteins may all be involved, and other mechanisms may exist that we still have to discover. Reducing hyperglycaemia by increasing glucose excretion may lead to improved insulin secretions to the extent that the other signs of insulin insufficiency are controlled without the need for insulin injections.
As cats typically suffer from diabetes that is like human type 2 DM, it is likely that they also suffer from glucose toxicity in the early stages of their DM. Reducing the hyperglycaemia may lead to improvements in insulin efficacy.
The SGLT2 is located in the proximal renal tubule and responsible for reabsorption of most of the glucose from the tubule in a diabetic animal. When blocked by SGLT2 inhibitors, glucose is not reabsorbed and glucosuria increases, which lowers blood glucose levels and slowly reduces glucose toxicity (Figures 1a and 1b).
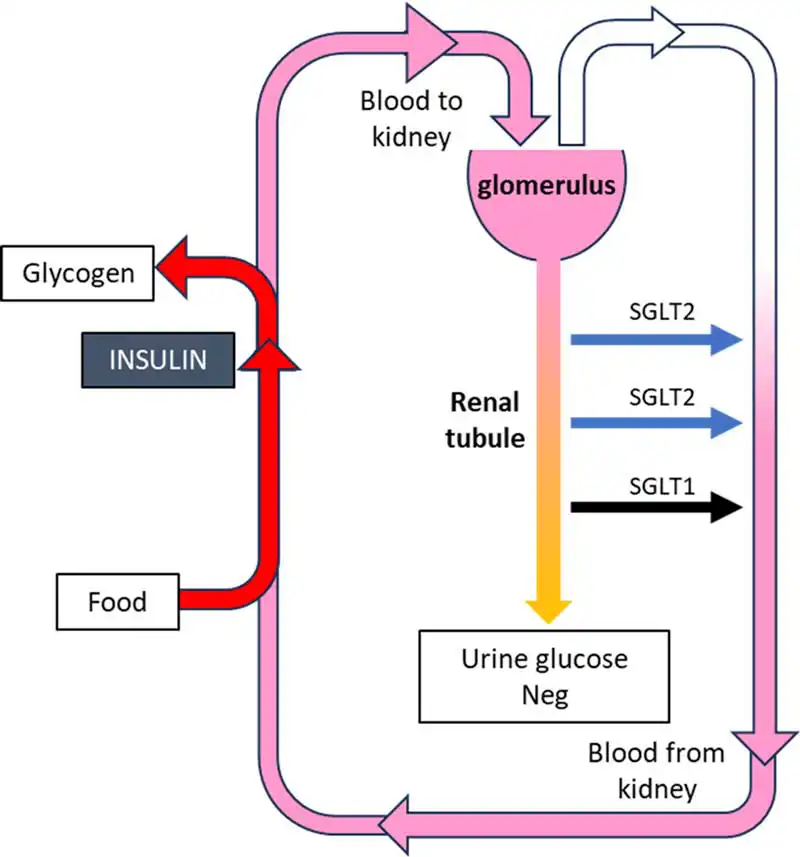
In two treatment trials with diabetic cats being treated with velagliflozin, the agent was effective in lowering glucose and fructosamine levels, and improving clinical signs such as polyuria/polydipsia1,2.
The overall treatment success was rated to be 84% in a cohort of newly diagnosed diabetic cats only treated with bexagliflozin – another SGLT2 inhibitor licensed for use in cats in the US3.
Velagliflozin is sold as a flavoured oral solution (Senvelgo; Boehringer Ingelheim) that can be administered either directly orally or with a small amount of food and is dosed based on bodyweight. This dose is fixed without the need for individual dose adjustments.
SGLT2 inhibitors can be used in newly diagnosed and pretreated cats, although switching stable diabetic cats on insulin to a SGLT2 inhibitor is not recommended, due to an increased risk of eDKA. The medication should not be used in cats with a previous history of diabetic ketoacidosis, current anorexia, lethargy, dehydration or cachexia.
In human medicine, SGLT2 inhibitors have shown efficacy in lowering blood glucose without an increased risk of symptomatic hypoglycaemia. The action of the sodium-glucose cotransporter 1 (SGLT1) present in the kidney, which is only mildly blocked by velagliflozin, will ensure sufficient glucose reabsorption to prevent hypoglycaemia. This risk is, however, increased if the SGLT2 inhibitor is used concurrently with insulin and so this approach is not recommended.
Velagliflozin does, to a certain extent, also block the SGLT1 in the small intestine. This leads to the development of diarrhoea in many patients at the beginning of treatment. Further side effects include polyuria/polydipsia that can be enhanced through the glucosuric effect of the SGLT2 inhibitor, dehydration, hypersalivation and urinary tract infections. The polyuria/polydipsia does subside with time to levels that are unlikely to worry most cat owners.
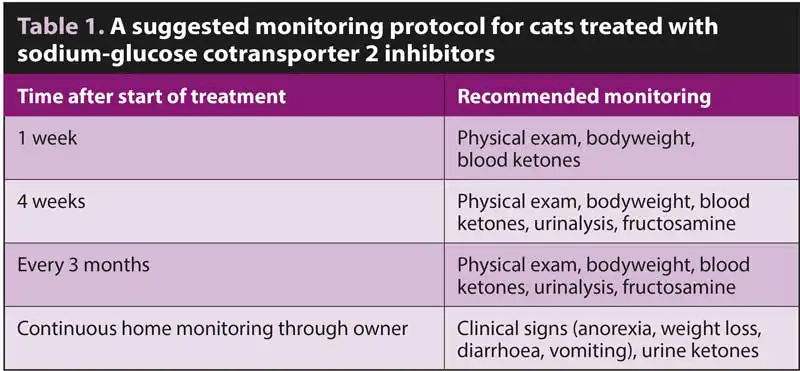
When using velagliflozin, veterinarians have to change their monitoring of diabetic cats, since urine glucose measurements will be persistently positive and blood glucose curves will no longer be useful or required. Especially in the first weeks after starting an SGLT2 inhibitor, it is, therefore, crucial that both veterinarian and owner monitor the cat closely for any adverse clinical signs, and determine ketone levels in the urine or in the blood.
Fructosamines – long since considered to be relatively poor at assessing the stability of insulin-treated cats – may actually be more useful in SGLT2-treated cats as their blood glucose is more stable.
It is essential that patients treated with SGLT2 inhibitors have sufficient residual beta cell function to prevent the development of diabetic ketoacidosis. This life-threatening complication will occur in cats that are not able to produce enough endogenous insulin to prevent ketogenesis.
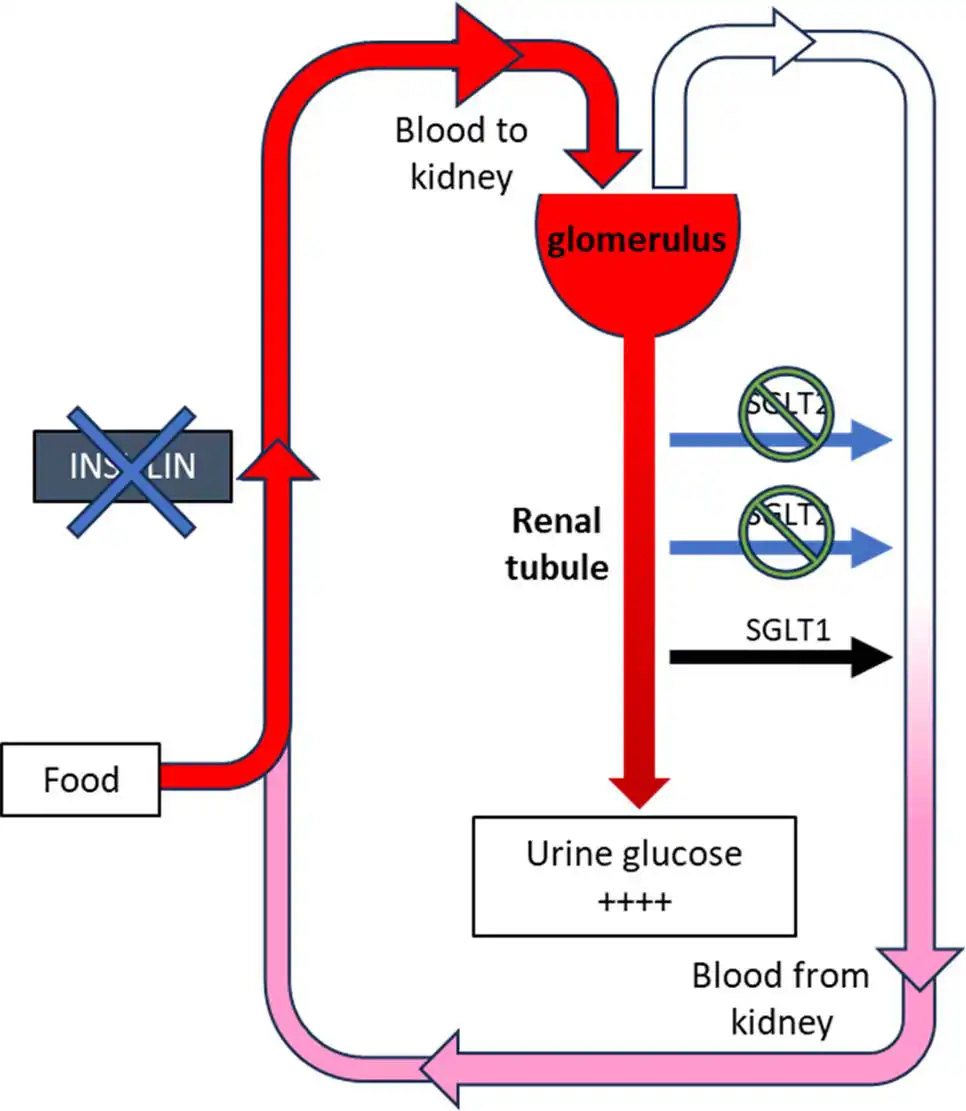
Euglycaemic diabetic ketoacidosis is a phenomenon almost exclusively seen in humans and cats treated with SGLT2 inhibitors that is characterised by typical clinical and biochemical abnormalities of diabetic ketoacidosis, but with normal glucose values. Normoglycaemia occurs through the action of the SGLT2 inhibitor, although the cat is insulin deficient.
About 4% to 7%1-3 of cats were reported to develop eDKA after having been started on a SGLT2 inhibitor. Within this group, 72% did so within the first seven days of treatment3, emphasising the importance of making the owner aware of clinical signs that may be associated with eDKA (lethargy, vomiting, anorexia and sudden weight loss, for example). Owners must monitor their cat closely and notify the veterinarian immediately in case of any concern. Home monitoring for ketonuria using a urine dipstick – especially in the first weeks after starting the SGLT2 inhibitor – is strongly recommended.
A suggested monitoring protocol is shown in Table 1.
Euglycaemic diabetic ketoacidosis is confirmed when the blood glucose is normal, but ketones are in either the urine (any detectable on a dipstick) or the blood (the threshold for betahydroxybutyrate that is measured with blood ketone meters is usually set at 2.4mmol/L; Figures 2a and 2b). Affected cats are usually lethargic, dehydrated and may be vomiting. However, even if clinical signs are not yet present, the situation is serious and warrants immediate hospitalisation.
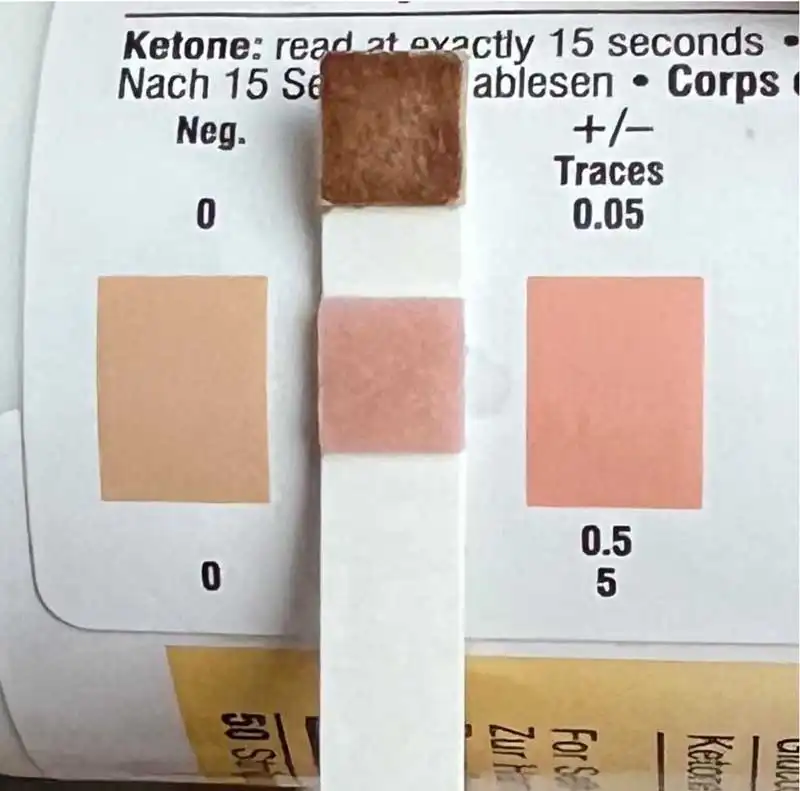
If eDKA is confirmed, the SGTL2 inhibitor must be stopped, and the cat treated with insulin immediately (despite the normal glucose levels). Glucose supplementation must be provided (and this will require higher doses than for hyperglycaemic diabetic ketoacidosis being treated with insulin), alongside the appropriate fluid therapy. In addition, adequate nutrition, if needed through a feeding tube, must be provided.
Restarting the SGLT2 inhibitor after eDKA is not recommended since the cat likely will not have sufficient endogenous insulin production to prevent further episodes of ketogenesis.
For many veterinarians, blood glucose curves are an important tool in monitoring DM in dogs and cats, allowing for evaluation of glycaemic fluctuations, the nadir and duration of insulin action. However, single-day blood glucose curves, both at home and in the hospital, can be both stressful for the pet and unreliable, with large variations between days. Furthermore, little agreement can be seen in the interpretation of blood glucose curves – even between experienced practitioners.
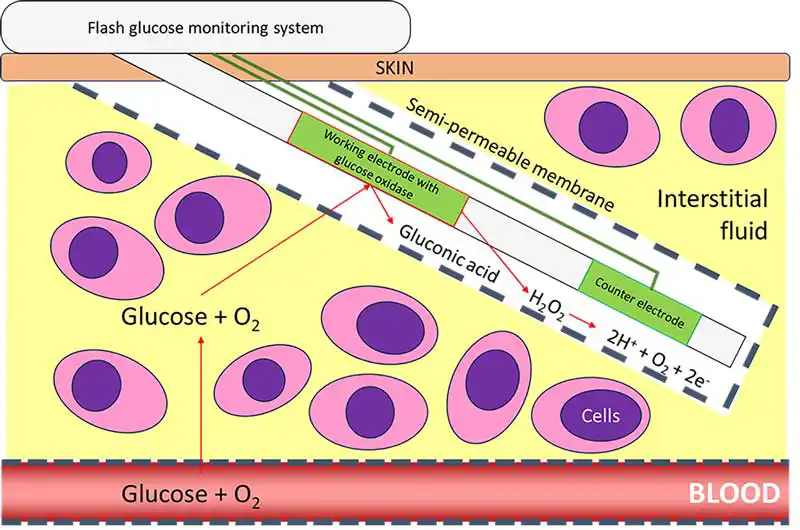
Continuous glucose monitoring devices can overcome some of these limitations and are advantageous in canine and feline diabetic management. These are minimally invasive devices that measure the interstitial glucose concentration in real time. The sensor consists of a platinum electrode and a glucose diffusion, limiting membrane containing glucose oxidase. In the presence of oxygen and interstitial glucose, an oxidative process leads to a current that is proportional to the glucose concentration in the interstitial space (Figure 3).
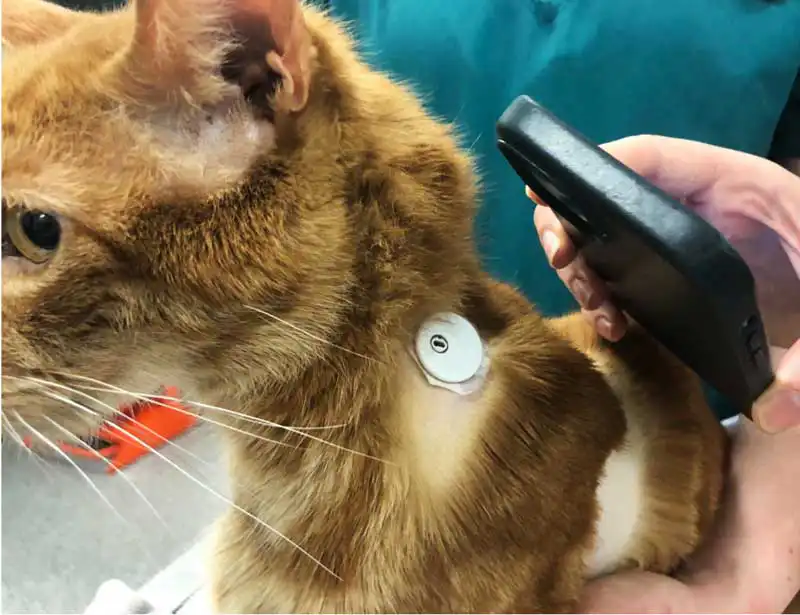
The latest version of the FreeStyle system Libre 3 (Abbott) has been approved for use in humans and currently represents the smallest sensor available. The size of a pound coin, it generates data for up to 14 days. The older, larger Freestyle Libre 2 is still available, but uses a different app. The Libre 3 allows for continuous real-time glucose readings delivered to the client’s smartphone every minute without the need for calibration. The latest glucose levels are transmitted when the smartphone scans the sensor (for this reason they are referred to flash glucose monitoring systems [FGMS]).
The sensor should be placed on a smooth skin surface that does not undergo too much movement. The area needs to be clipped and the skin cleaned with alcohol and dried. The sensor is then attached to the skin by pressing and holding it firmly in place for 30 seconds. If needed, a small amount of tissue glue can be applied. The sensor can be covered by a cotton shirt or surgical vest, but tight coverings should be avoided since the sensor needs oxygen supply to function.
The owner should download the appropriate app and activate the sensor with their mobile phone (Figure 4). One hour later it starts working. The owner must be made aware that the patient is not allowed to swim while the sensor is in place, but mild raindrops are tolerated.
Generally, patients tolerate the application very well and only very rarely is sedation needed. A mild erythema at the site of the sensor application has been recorded in dogs and cats, with 6% of cats and 3% of dogs developing more serious complications such as skin erosions, severe crusting or abscess formations4,5. The possibility for reduced sensor lifespan due to premature detachment should be discussed with the owner prior to the application, since especially cats can be masters at removing them.
The overall clinical accuracy is generally considered adequate for dogs and cats using the aforementioned devices, although some limitations need to be considered. Flash glucose monitoring devices are less accurate in the hypoglycaemic range and not as reliable in detecting rapid changes in blood glucose concentrations, which can be explained by the time lag that comes with the equilibrium between interstitial and blood glucose. These devices should, therefore, not be used for immediate treatment decisions.
Severe dehydration – for example, in patients with diabetic ketoacidosis – can affect the reliability of the device, so rehydration is generally advised before placing the device. Glucose readings – especially those in the hypoglycaemic range – should always be judged in light of clinical signs, and evaluation of the general pattern rather than individual measurements is recommended. If unexpected glucose values or those that are not consistent with the clinical picture are obtained, blood glucose should be measured for validation.
A study has evaluated the impact the FGMS have on the owner’s quality of life6. A total of 80% of owners considered the device easier to use and less stressful than traditional blood glucose curves and overall, 95% reported their pet had better glycaemic control6. The most common challenges for owners included proper sensor fixation, preventing premature detachment and costs6.
Biomarkers can be targeted to improve the diagnosis, monitoring and prognosis of various diseases. For canine DM, the biomarker of choice has historically been serum fructosamine, which represents the average blood glucose concentration of the preceding one to three weeks. In humans, however, the longer-term marker haemoglobin A1c (HbA1c) is universally adopted as a measure for the diagnosis, monitoring and prognosis of both type 1 and type 2 DM.
Haemoglobin A1c is a marker for the average glucose concentration during the lifespan of erythrocytes, which is 110 days in dogs. It is formed by the non-enzymatic, insulin-independent, and irreversible binding of glucose to haemoglobin in circulating erythrocytes. A moderate positive correlation exists between HbA1c and fructosamine in dogs7.
In the UK, canine HbA1c can be measured in a whole EDTA blood sample using an immunoturbidimetric assay8 with the value expressed in mmol/mol. No test for feline HbA1c is currently commercially available in the UK. When interpreting this marker, the concomitant haematocrit or haemoglobin should be taken into account since diseases that lead to anaemia or polycythaemia can affect HbA1c measurements.
HbA1c can be of assistance in both diagnosing and monitoring canine DM. Its concentrations have been demonstrated to be significantly higher in diabetic dogs compared to non-diabetics9 with its diagnostic accuracy being superior to serum fructosamine10. Since it reflects the average glucose concentration of the preceding two to three months, in the early stages of DM HbA1c can still be normal while fructosamine might already be increased.
The overall value of HbA1c for assessment of glycaemic control in diabetic dogs was considered rather poor and only a weak-to-moderate negative correlation was noted between HbA1c values and clinical score in one study10. Importantly, large inter-individual differences in glycation can be seen, and this marker should be rather considered useful for monitoring individuals over time than comparing individuals or setting strict cut-offs for “good control” versus “poor control” (Figure 5).
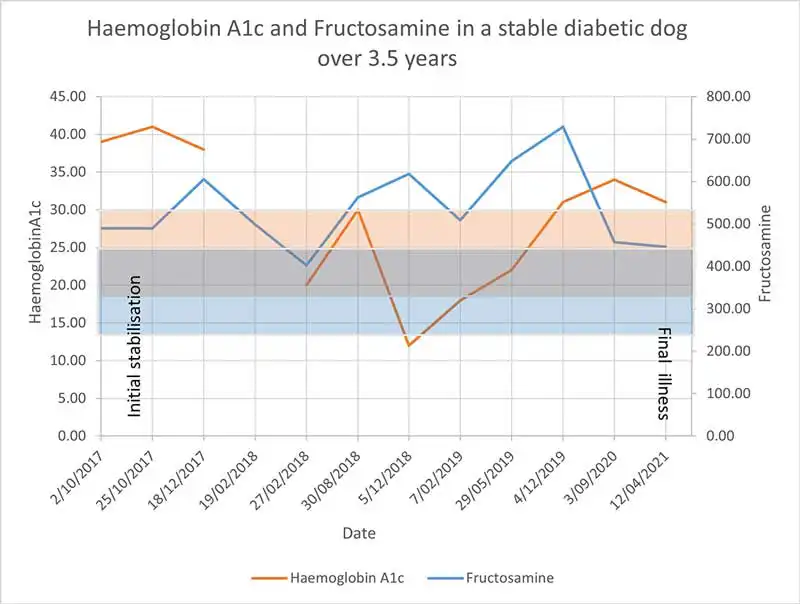
Because HbA1c represents a long-term marker for glycaemic control, it should only be measured every two to three months. Although it can be of great value as an additional marker, HbA1c should not be used as a sole indicator for glycaemic control and always be interpreted in light of history, clinical signs and other biochemical abnormalities. Little is known regarding the prognostic significance of HbA1c in diabetic dogs, but in one study, it was not correlated with overall survival (Kraemer et al, unpublished data).
Similarly to human medicine, HbA1c promises new insights into canine DM, and although it is expected to increasingly complement diabetic management in the coming years, studies are still ongoing.
Velagliflozin represents a very promising novel approach to diabetic cats. The advantages of once-daily oral administration without the need to titrate dose are significant, but every veterinarian using it must be aware of the (unique) risks associated with it. Euglycaemic ketoacidosis is a serious condition and vets must know how to detect and deal with it when it arises.
FGMS provide a convenient method of assessing average glycaemic response patterns to insulin injections and the overall clinical accuracy is adequate. However, they come with some limitations such as in dehydrated animals and should not be used for making immediate clinical decisions.
HbA1c provides an assessment of glycaemic control over the preceding two to three months and, therefore, is likely to be more suitable for routine monitoring of stable diabetic dogs than fructosamines. Values should be compared within individual dogs rather than using generic cut-offs. Increasing values would suggest a re-appraisal of the diabetic management protocol, rather than simply increasing doses of insulin.