19 Dec 2016
Diagnosis and management of canine lymphoma on a budget
Ana Lara looks at defining the essential versus desirable aspects of treating this common type of cancer in dogs, to help keep costs down for owners.
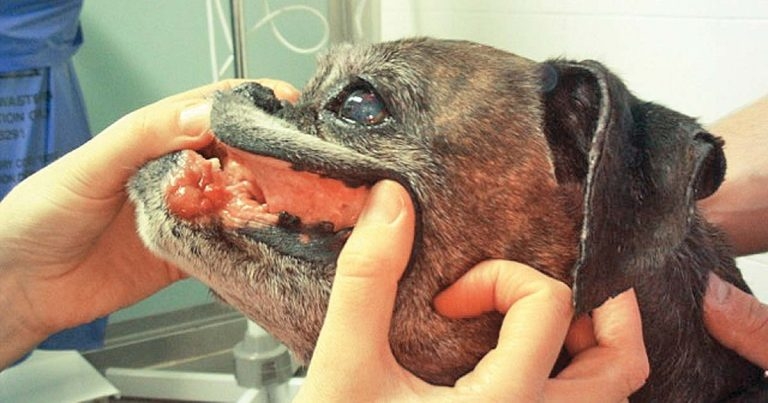
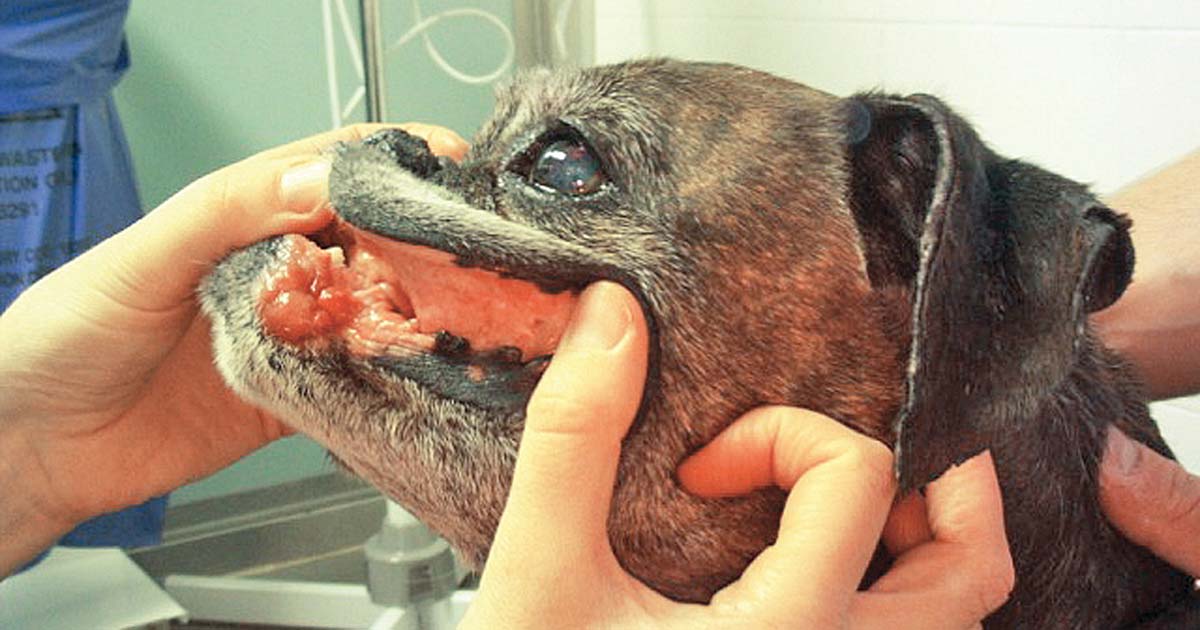
Figure 2. An example of localised oral mucocutaneous lymphoma.
Lymphosarcoma (LSA) is the most common canine cancer type and the number one canine haematopoietic malignancy diagnosed in practice. As a chronic disease, most dogs with LSA will receive some form of therapy for their life; so, as a general rule, the most successful cases will require long-term investment.
Research has demonstrated the prognostic importance of gathering as much information as possible regarding morphologic classification and immunophenotype in canine lymphoma. Sometimes clients might have a limited budget and it is useful to tailor planned tests based on the prognostic information or relevancy towards treatment decision making they can provide. This approach allows optimisation of the economic resources available towards therapy for the specific type of LSA in our canine patient.
Around 70% of cases have a multicentric presentation with involvement of peripheral lymph nodes plus or minus spleen, liver and bone marrow or other internal organs, but it can arise at any anatomical part. Less commonly, LSA arises in localised anatomical locations (such as skin, eye, liver, gastrointestinal tract, nasal and splenic)1.
Despite being a rapidly progressing systemic neoplasm, treatment is usually satisfactory as most patients respond well to chemotherapy. Although rarely curable, quality of life is maintained or improved during treatment. It has, therefore, become common practice for an increasing number of vets to diagnose and treat this disease.
Is it worth investing in lymphoma diagnosis?
Histologic classification (Panel 1) and grade, associated with mitotic index, are known to be prognostic independently of the type of therapy chosen and are both usually obtained on histopathology2. Depending on the lymphoid cell of origin, LSA can be B cell, T cell or NK cell LSA (immunophenotype). High-grade T-cell and NK immunophenotype have been associated with shorter remission and survival times3.
Also, some specific types of LSA might not need therapy or be more resistant to some types of drug. This important prognostic information makes refining the diagnosis of LSA well worth the investment.
If diagnosis is performed on a lymph node biopsy, the pathologist can provide morphologic classification and immunophenotype should be obtained with immunohistochemistry. In practice, diagnosis often relies on a cytologic specimen as it is cheaper and results are quickly available. We can obtain important prognostic information with a cytomorphologic interpretation, together with the immunophenotype4.
Flow cytometry (using a liquid suspension of fine needle aspirates) provides immunophenotype and a comprehensive panel of all lymphoid surface receptors present in the neoplastic population – being available through several diagnostic laboratories across the country, which will advise you regarding what media to use for sample submission. Alternatively, requesting PCR for antigen receptor rearrangement in an existing cytology can inform about B cell versus T cell type.
B-cell neoplasms
- Precursor B-cell neoplasms
- Precursor B lymphoblastic leukaemia/lymphoma
- Mature (peripheral) B-cell neoplasms
- B-cell chronic lymphocytic leukaemia/prolymphocytic
- Leukaemia/small lymphocytic lymphoma
- B cell prolymphocytic leukaemia
- Lymphoplasmacytic lymphoma
- Splenic marginal zone B-cell lymphoma
- Plasma cell myeloma/plasmacytoma
- Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type
- Nodal marginal zone lymphoma
- Follicular lymphoma
- Mantle cell lymphoma
- Diffuse large B-cell lymphoma
- Mediastinal large B-cell lymphoma
- Burkitt’s lymphoma/Burkitt’s cell leukaemia provisional entity: high-grade B-cell lymphoma Burkitt’s-likea
- Primary effusion lymphoma
T-cell and putative natural killer cell neoplasms
- Precursor T-cell neoplasm
- Precursor T lymphoblastic
- Lymphoma/leukaemia
- Mature (peripheral) T-cell and natural killer cell neoplasms
- T-cell prolymphocytic leukemia
- Large granular lymphocyte leukaemia
- Aggressive natural killer cell leukaemia
- Peripheral T-cell lymphomas, unspecified
- Adult T-cell lymphoma/leukaemia
- Intestinal T-cell lymphoma (+enteropathy associated)
- Hepatosplenic ɣδ T-cell lymphoma
- SC panniculitis-like T-cell lymphoma
- Mycosis fungoides/Sézary syndrome
- Anaplastic large cell lymphoma, T and null cell primary cutaneous type
- Peripheral T-cell lymphoma not otherwise specified
- Angioimmunoblastic T-cell lymphoma
- Angiocentric T-cell lymphoma
Immunophenotype, grade and prognosis
High-grade T cell LSA with an aggressive clinical course independent of treatment regimen (median survival times; MST 159 days), present on flow cytometry CD45+ and class II major histocompatibility complex (MHC-) expression5. Multicentric high grade T cell LSAs are often inherently resistant to doxorubicin6, which would explain the poorer responses and shorter remission rates described when treated with cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine and prednisolone (CHOP)-type protocols7.
However, their survival times can be comparable to B cell LSAs when treated with mustargen, oncovin, procarbazine and prednisone (MOPP) or lomustine, vincristine, procarbazine and prednisolone (LOPP) protocols, including alkylating agents such as lomustine, procarbazine or chlormethine8,9. Low-grade multicentric T cell LSAs (usually classified as T zone LSA on histology) have a distinctive flow cytometry profile (CD45- and MHC class 2+) with median survival times (MST) of 637 days. Use of chemotherapy does not affect their prognosis, so treatment might not be necessary2,10.
The most common morphologic type of multicentric LSA is diffuse large B cell LSA (DLBCL), which can be immunoblastic or centroblastic, and their reported MSTs are 308 and 213 days, respectively, with improved prognosis compared to other types of high grade B cell LSA (MST of 160 days)2. Dogs with B cell LSA have been traditionally treated with CHOP-type protocols, but it seems MST for DLBCL induced with CHOP or cyclophosphamide, oncovin and prednisone or prednisolone (COP) can be similar11.
Anatomic forms and prognosis
Specific anatomic presentations of canine LSA can have an improved prognosis, compared with multicentric LSA, or might benefit from a completely different type of therapy. Splenic marginal zone LSA is an indolent form of B cell LSA primarily affecting the spleen, often without additional organ involvement. The diagnosis requires histopathology and immunophenotyping. MSTs with splenectomy is 383 days and for asymptomatic dogs, 1,153 days. Lymph node metastasis, haemoabdomen, anaemia postoperative
adjuvant chemotherapy and concurrent malignancy did not affect survival. This positive prognosis was also reported for splenic mantle cell LSA. After surgery, no need for systemic treatment seemed to exist in these cases12,13.
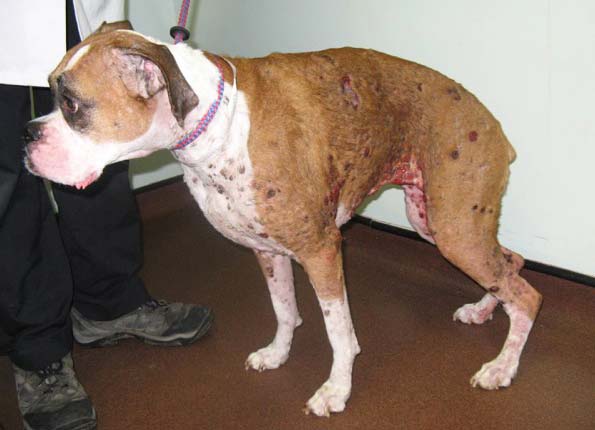
Cutaneous epitheliotropic LSA (Figure 1), usually of T cell origin and low grade, presents diffuse involvement of the skin at times with peripheral lymphadenopathy14. Longer-lasting responses occur with single agent oral lomustine and prednisolone, and evidence supports considering oral masitinib treatment at relapse15.
If only solitary lesions present, surgery or radiotherapy can achieve disease control. For example, dogs with localised oral mucocutaneous LSA (Figure 2) without lymph node involvement have MSTs of 770 days with radiation therapy16.
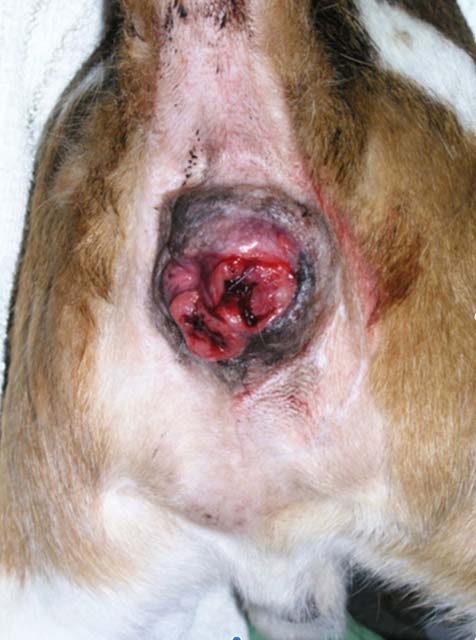
Alimentary LSA in dogs presents with predominantly diffuse involvement of the gastrointestinal tract. Prognosis even with CHOP-type chemotherapy protocols is poor, with MSTs of 77 days17. On the other hand, if LSA is confined to the large bowel (colon, rectum or both; Figure 3), the prognosis with chemotherapy is excellent, with median remission periods of 1,318 days and MSTs of 1,845 days. No difference is observed using COP versus CHOP protocols, making it appealing to use COP as it is cheaper and has less adverse effects on prevalence18.
Staging – does it really help with treatment decision making?
Expenses can be a limiting factor to perform staging. This is important when the cost of these tests could affect the owner’s subsequent ability to afford the treatment, so it is useful to be aware of the situations where staging would have an impact in prognosis and treatment approach.
The minimum database required before starting therapy in canine LSA is a complete blood count, including blood smear evaluation, serum biochemistry and urinalysis. Complete clinical staging consists of imaging of thorax and/or abdomen to assess the extent of the disease (Panel 2), plus or minus internal organ sampling.
Anatomic site
A Generalised
B Alimentary
C Thymic
D Skin
E Leukaemia (true)*
F Others (including solitary renal)
Stage (to include anatomic site)
I Involvement limited to a single node or lymphoid tissue in a single organ¥
II Involvement of many lymph nodes in a regional area (± tonsils)
III Generalised lymph node involvement
IV Liver and/or spleen involvement (± stage III)
V Manifestation in the blood and involvement of bone marrow and/or other organ systems (± stages I to IV)
Each stage is sub-classified into:
a Without systemic signs
b With systemic signs
*Only blood and bone marrow involved
¥Excluding bone marrow
In general, dogs with multicentric LSA, not sick (substage a), haematology and blood chemistry without clinically relevant abnormalities are likely to be classified as stage III or IV, in which case the prognosis and treatment are similar. Staging would be particularly useful for non-multicentric LSA when all disease is internal (Figure 4)1.
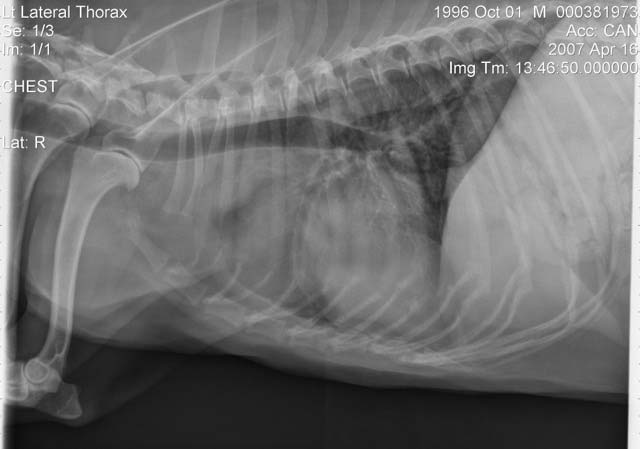
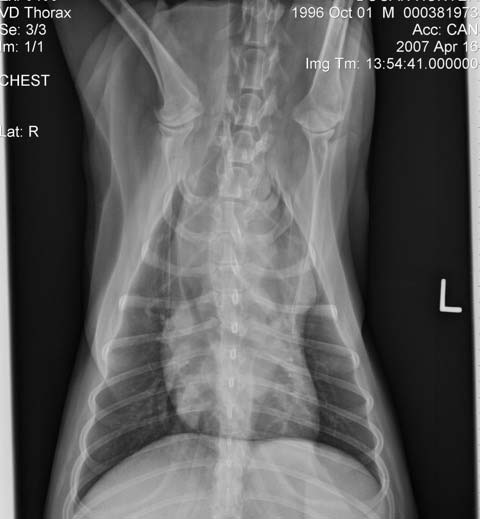
A third of dogs with multicentric LSA have bone marrow infiltration (stage V) at the time of diagnosis, which correlates with shorter MSTs when treated with multi-agent chemotherapy protocols. Bone marrow infiltration can be determined if malignant circulating cells are observed in the blood smear or can be suspected if there are peripheral blood cytopenias. If the latter is the case, bone marrow aspiration will demonstrate infiltration or rule in/out immune mediated cytopenia secondary to LSA. PCR for antigen receptor rearrangement in peripheral blood can detect circulating neoplastic cells without additional value in performing a bone marrow aspirate and saving costs19.
At a practical level, if clinically relevant cytopenias do not exist, such as severe neutropenia or thrombocytopenia, it is unlikely this stage will change clinical management. If the dog is severely neutropenic, wide-spectrum antibiotics and non-immunosuppressive chemotherapy with L-asparaginase will often be the first step, followed by the protocol chosen until neutrophil count recovers. It is generally important to be aggressive as adequate production of platelets and white and red blood cells will not be re-established until a large percentage of malignant cells are eliminated from the bone marrow. Some studies advocate for a benefit in the use of infusions of cytosine arabinoside as part of the induction protocols for stage V LSA20.
Dogs with substage B (unwell at diagnosis) have a worse prognosis, likely due to a more advanced presentation or critical involvement of body systems.
Establishing disease extension can help us tailor the induction treatment to avoid increased adverse effects or further deterioration. Imaging of the thorax can evidence presence of mediastinal masses or pleural effusion in dogs with respiratory signs and be used to obtain diagnostic samples if no external signs of disease present. Drugs known to have a fast effect, such as L-asparaginase and dexamethasone, can be used to start induction, leading to a marked improvement with minimal handling and helping minimising hospitalisation time.
When gastrointestinal signs exist, presence of focal high-grade LSA in the form of intestinal or gastric masses should be ruled in/out. In this scenario, severe risk exists of ulceration or perforation17. A disrupted gastrointestinal mucosa is already present and when chemotherapy is started, a large number of tumour cells infiltrated in the gut or stomach will be rapidly killed. To avoid further deterioration, intense gastrointestinal symptomatic therapy should be in place prior to treatment. Fast-acting drugs (L-asparaginase) should be avoided and drugs with less prevalence of GI toxicity should be considered.
In dogs that present liver dysfunction, symptoms such as moderate to marked increase in serum alanine aminotransferase activity and/or bile acids, abdominal ultrasound and hepatic fine needle aspiration would be useful to determine whether malignant liver involvement or concomitant hepatic disease is apparent. Some drugs used to treat LSA require hepatic activation (for example, cyclophosphamide and lomustine) and often hepatic inactivation (for example, vinca alkaloids and alkylating agents).
As a result of hepatic metabolism impairment, reduced drug efficacy can occur or increased adverse effects if drug clearing is decreased. If hepatic or severe hepatic involvement exists, we can consider dose reductions and avoid alkylating agents until hepatic function is restored.
Which protocols can fit a tight budget when treating lymphoma?
Multi-agent protocols are the treatment of choice for multicentric canine LSA inducing remission in 60% to 90% of dogs and survival rates of one year in around 50% of dogs, 20% of which may survive for up to two years. MSTs usually range from 6 to 14 months depending on the immunomorphologic type of multicentric LSA1.
If on a tight budget, COP-type protocols (a combination of cyclophosphamide, vincristine and prednisone) with a short induction of eight weeks are, in general, affordable, and adverse effect prevalence and monitoring requirements are lower than for CHOP or LOPP protocols. Oral maintenance therapy with chlorambucil, methotrexate and prednisone is a reasonable option after induction is finished21.
Median remission times of six months described are shorter than for CHOP protocols (median remissions of 10 to 11 months),
which have longer inductions – typically 19 to 25 weeks for the widely used Madison Wisconsin protocol21,22.
Single drug protocols in combination with steroids can achieve a good response, but remissions are shorter than with multi-agent protocols due to rapid development of resistance. Doxorubicin administered every three weeks provides response rates of 60% to 75% and median remission periods of four to five months. Prednisolone, as a single agent, induces remission of one to three months in around 50% of patients. Other drugs with proven efficacy as single agents in the treatment of canine LSA are cyclophosphamide, L-asparaginase, epirubicin, mitoxantrone, actinomycin D and lomustine1, 23-26.
Most dogs with LSA will come out of remission and the overall survival will depend on establishment of therapy at this point. If the patient has relapsed when in maintenance treatment or not in treatment, it is appropriate to again use the initial induction protocol. A study showed survival times in dogs with B cell LSA were similar for dogs treated with COP and rescued with doxorubicin protocols than for dogs treated with Madison Wisconsin protocols at induction and relapse11.
Within a tight budget, all the aforementioned single agents could be used as a rescue protocol, with continued oral lomustine after a single dose of L-asparaginase or single agent doxorubicin being the most efficacious single agents in this setting.
- Some drugs mentioned in this article are used under the cascade.
