14 Sept 2020
Evaluating haematuria and dysuria via urine cytology
From the <em>Vet Times</em> Diagnostic Dilemmas series – Francesco Cian focuses on the case of a 10-year-old mixed‑breed dog that presented for evaluation of haematuria and dysuria.

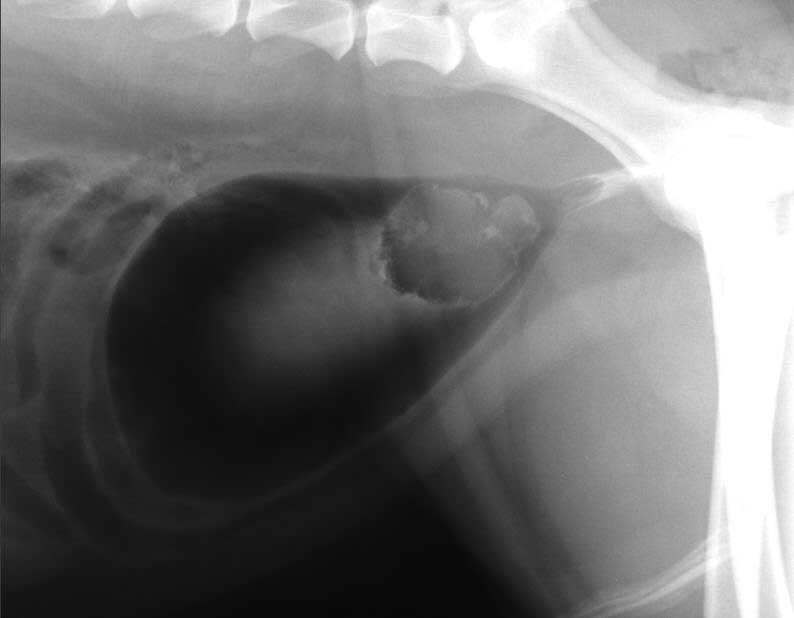
Figure 1. Right lateral view double contrast cystogram of the caudal abdomen of a dog with haematuria and dysuria. Image: Mauro Pivetta
Jack is a 10-year-old mixed‑breed dog presented for evaluation of haematuria and dysuria that have been persisting for several weeks, and had only temporary and partial resolution after antibiotic therapy.
Right lateral view double contrast cystogram of the caudal abdomen showed a well-defined, irregularly marginated filling defect centred in the urinary bladder trigone, highly suggestive of neoplasia (Figure 1).
A urine sample obtained by traumatic catheterisation was collected and sent to an external laboratory for cytological examination.
The aspirate harvested a main population of pleomorphic and polygonal cells often forming variably sized clusters on a clear background (Figures 2 and 3).
Cells show moderate amounts of basophilic cytoplasm with defined borders. Occasionally, they contain round, eosinophilic, large inclusions (Melamed‑Wolinska bodies) that sometimes displace the nucleus to the peripheral of the cell (Figure 2; red arrow).
Nuclei are mainly round – sometimes oval or slightly irregular – and are variably located within the cell. Chromatin is coarse granular and small multiple round nucleoli are often seen.
Anisocytosis and anisokaryosis are moderate, sometimes marked (Figure 3; black arrow). Rare mitotic figures are visible (Figure 3; green arrow). Leukocytes are extremely rare and are not supportive of inflammation.
Diagnosis
A diagnosis of transitional cell carcinoma (TCC) was given.
Insight
TCC – also referred to as urothelial carcinoma – is the most common form of malignant neoplasm of the urinary tract in dogs, accounting for 50% to 75% of reported cases of canine urinary bladder cancer.
The aetiology is not completely understood, but is likely to be multifactorial and prevalence appears to be higher in certain breeds – in particular Scottish terriers, which have a 21-fold increased risk.
Dogs usually present with lower urinary tract signs (for example, haematuria, stranguria, pollakiuria and dysuria), and many have concurrent urinary tract infections (UTIs) that may initially delay the diagnosis. Resolving the infection may temporarily alleviate the clinical signs, and patients often present with a several-month history of lower urinary tract signs.
Grossly, TCC usually presents as a solitary papillary, polypoid or sessile mass. The most common location in dogs is the trigone of the urinary bladder, but this neoplasm can also arise from the renal pelvis, ureters, urethra or prostate.
In a necropsy study of 137 dogs with TCC (Fulkerson and Knapp, 2015), nodal and distant metastases were found in 42% and 58% of dogs, respectively. The lung was the most common site of distant metastases, followed by other abdominal organs, bones (for example, lumbar spine) and skin.
From a diagnostic point of view, urine sediment examination reveals neoplastic cells in a limited percentage of cases and is unlikely to provide a definitive diagnosis.
Direct fine-needle aspiration of the mass is the technique providing the highest cellular samples for analysis, although the potential for seeding neoplastic cells is a consideration. Therefore, traumatic catheterisation (also called suction biopsy) is often preferred.
When cells have a typical transitional morphology, exhibit marked criteria of malignancy and no evidence of inflammation or infection exists, a diagnosis of TCC can be easily achieved by cytology. This may not be possible when neoplastic cells are well differentiated or in the presence of concurrent inflammation since morphology of well‑differentiated neoplastic cells and hyperplastic/dysplastic transitional cells may overlap.
In those cases, further diagnostic investigations should be considered, and may include histopathology and/or BRAF mutation testing.
What’s new?
Conventionally, biopsy and histopathological evaluation are required to confirm a diagnosis of canine TCC, and are particularly important when a definitive cytological diagnosis is not possible.
More recently, new non‑invasive techniques have been described and have shown very promising results. These include molecular testing for BRAF mutation, which is a PCR-based assay that searches for a single mutation in a gene called BRAF within transitional epithelial cells.
This mutation has been identified in up to 85% of TCC cases and to date this has never been recorded in urine specimens, neither from healthy dogs nor in patients with inflammatory or dysplastic processes affecting the genitourinary tract.
This means that finding the BRAF mutation is confirmatory for TCC, but the lack of it does not entirely rule it out, since false negative results may occur.
One of the main advantages of this test is that it can be performed on almost any diagnostic sample that contains transitional epithelial cells, including pre-stained cytology smears with equivocal diagnosis, which means no additional samples are required.
Molecular testing for BRAF mutation may also be considered as a screening test in free-catch urine samples of healthy dogs with an increased risk of developing TCC, likely enabling identification of these cases at early and even preclinical stages of the disease.
Once a dog has been diagnosed as positive for BRAF mutation and shown to have TCC, repeat analysis may also be used over time to monitor changing levels of the mutational load detected in free-catch urine during treatment.
Take-home message
Definitive diagnosis of transitional cell carcinoma can be achieved by cytology. This is facilitated when transitional cells exhibit marked cytological features of atypia, no concurrent inflammation exists and evidence of a distinct mass exists.
When definitive cytological diagnosis is not possible, further diagnostic investigations should be considered and may include histopathology or other non-invasive techniques, such as molecular testing for BRAF mutation.
