31 Aug 2021
Sam Taylor and Emi Barker summarise thoughts on this disease’s presentation and diagnosis, plus introduce a new era of treatment.


Figure 1. The “classic” FIP cat with a large volume abdominal effusion. Image: the Feline Centre, Langford Vets, University of Bristol
FIP is caused by virulent mutations of feline coronavirus (FCoV) that transform it from a mild and enteric infection into a serious systemic disease.
Like other coronaviruses, FCoV is a large, enveloped RNA virus – this is important when we consider immune system evasion, environmental survival, detection, treatment and prevention. FIP has a high mortality rate and, until very recently, treatments have been relatively ineffective.
This article summarises current thoughts on presentation and diagnosis. It also introduces a new era of treatment of FIP, with the recent availability of legal medications in the UK.
FCoV is an Alphacoronavirus found to infect domestic cats and other felids. It is from the same genus as canine enteric coronavirus and transmissible gastroenteritis virus of pigs. FCoV cannot infect humans and is only distantly related to SARS-CoV-2, a Betacoronavirus and the causative agent of COVID-19.
FCoV, as the biotype feline enteric coronavirus (FECV), is commonly detected in the faeces – particularly from cats living in multicat households. Infection is typically acquired via the faecal-oral route when kittens or young cats become exposed to shedding cats. As an enveloped virus, environment survival is generally poor, unless encased in faeces, and it is susceptible to most disinfectants.
In some cats and at some point following initial infection – between viral replication in enterocytes, and efficient replication in macrophages and monocytes – the less-virulent FECV mutates into the virulent form associated with FIP – that is, the biotype FIP virus (FIPV). Certain mutations involved in this transition have been found in the spike protein gene, although none are yet pathognomonic for FIP.
The high frequency of genomic mutation – a feature of RNA viruses – may also facilitate host-immune response evasion and drive tissue tropism, leading to the various disease manifestations. Natural direct transmission of FIPV between cats is thought to be rare, with FIPV – and consequently FIP – generally believed to arise as a result of a de novo mutation in an individually FCoV-infected cat.
One of the many complexities of FCoV and FIP is that the infection manifests in many different ways depending on viral factors such as strain and dose, but also the cat’s immune response and genetic factors.
A strong cell-mediated immune (CMI) response towards FCoV appears to provide protection against FIP. In contrast, cats mounting a predominantly antibody-mediated response, with a weak CMI response, typically succumb to effusive “wet” disease due to immune-mediated vasculitis, while intermediate cats develop the tissue granulomas typical of non-effusive “dry” disease.
It is important to remember that huge overlap exists between the effusive and non-effusive forms of FIP, and these manifestations should be considered as a spectrum; brief episodes of effusive disease can occur before a non-effusive form predominates and conversely, in the terminal stages of a non-effusive form, effusions may form. Additionally, many cats with effusions have tissue granulomas.
The classic presentation of a young cat with protein-rich ascites (Figure 1) may offer a more straightforward diagnosis; however, other cats may prove more of a diagnostic challenge. Non-specific signs are common, including lethargy, anorexia and weight loss.
Affected cats may be febrile, with a moderate fever (typically less than 40°C, which is often fluctuating and poorly responsive to NSAIDs or antimicrobials) and icterus when present is usually mild (Figure 2).
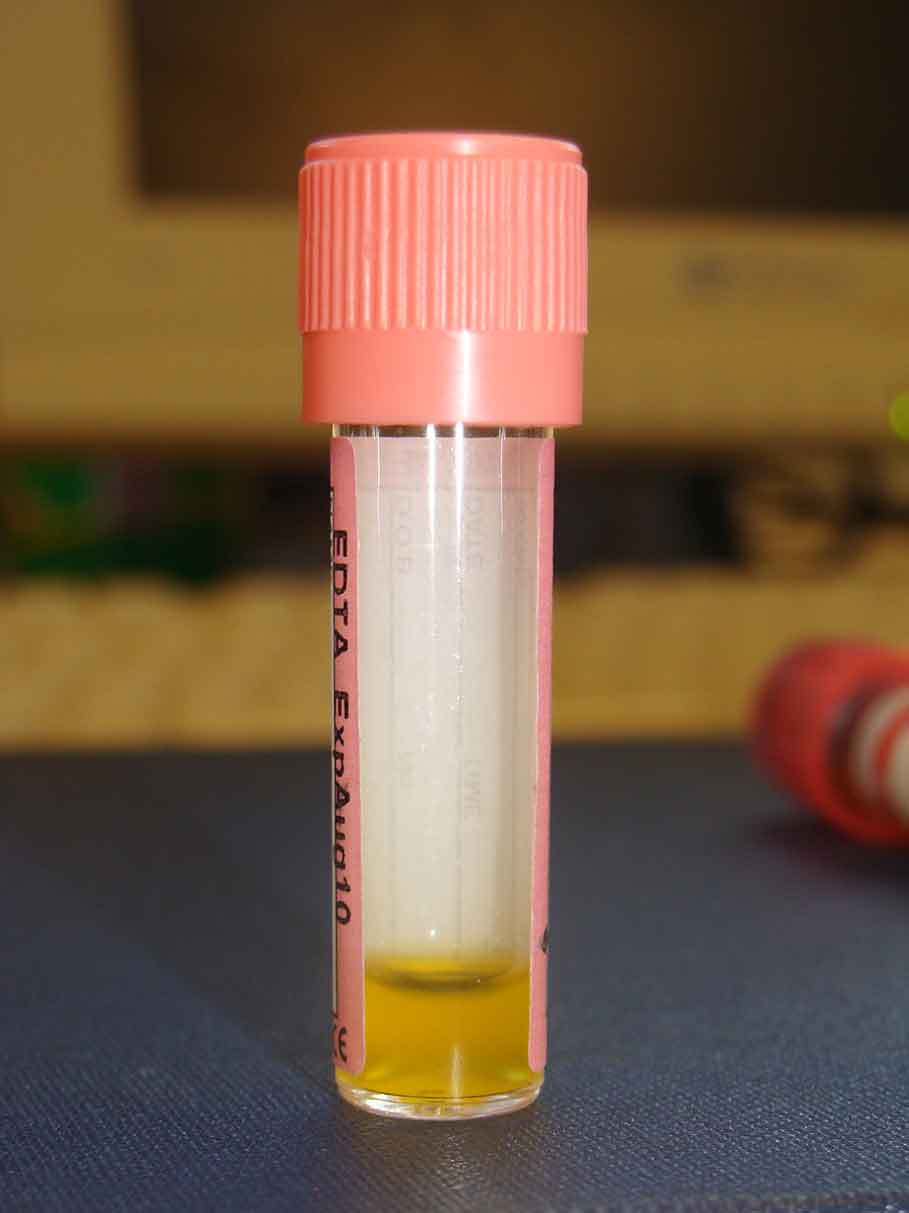
Viscous effusions with high protein levels form in approximately 80% of cats with FIP – most (approximately 85%) involving the abdominal cavity, with fewer having thoracic cavity (approximately 20%) involvement.
Pericardial effusions are occasionally seen, albeit rarely causing tamponade, and scrotal effusions may occur, albeit rarely, in entire cats.
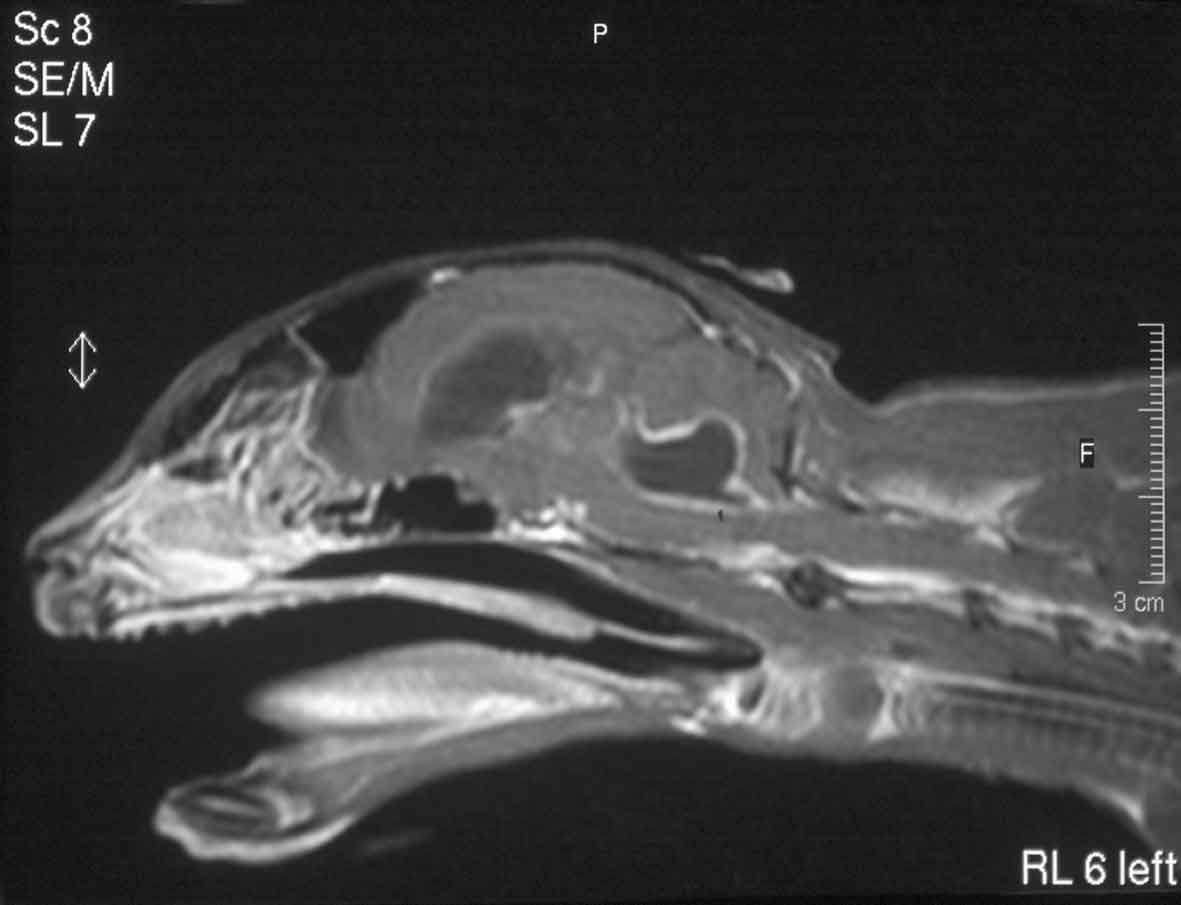
Pyogranulomatous lesions may occur in any tissue, and while abdominal organs (for example, mesenteric lymph nodes and kidneys) are commonly involved, disease may be restricted to other organs, such as the eyes, brain or spinal cord.
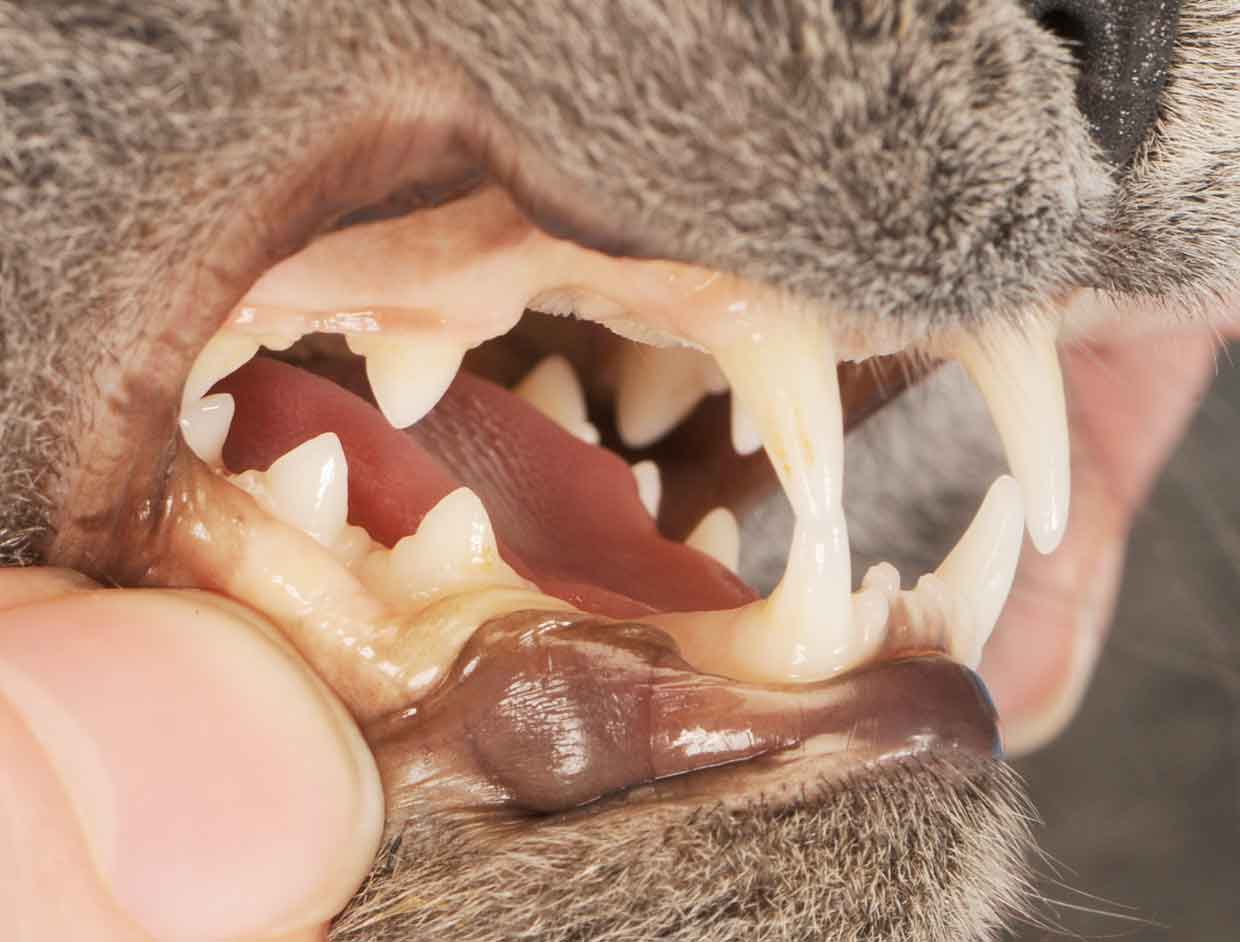
Ocular signs include uveitis, keratic precipitates, hypopyon, hyphaema (Figure 3) and retinitis. Neurological signs include ataxia, seizures, nystagmus, hyperaesthesia and behavioural/mentation changes.

Although one abnormality alone does not make a diagnosis of FIP, nor their absence rule it out, the clinician may base increasing suspicions of FIP on the following findings, bearing in mind signalment, clinical picture, clinical pathology and imaging results in the absence of an alternative, more likely explanation:
High levels of FCoV antibodies only indicate previous FCoV infection and are not diagnostic of FIP
A definitive diagnosis of FIP is confirmed with positive immunostaining for coronavirus antigen in macrophages associated with pathological changes of FIP in formalin-fixed tissue samples. However, collection of samples for histopathology and immunostaining require invasive procedures, which may be contraindicated in a sick cat.
Alternatively, the presence of coronavirus antigen-positive cells in cytological specimens (effusion, aqueous humour, CSF cytospin preparations or FNAs from any abnormal organs – for example, mesenteric lymph node) showing pyogranulomatous changes is very supportive of a diagnosis and may allow samples to be collected less invasively.
Some researchers have used cell pellets prepared from centrifuged effusion samples to improve the sensitivity of immunostaining (Tasker et al, 2021).
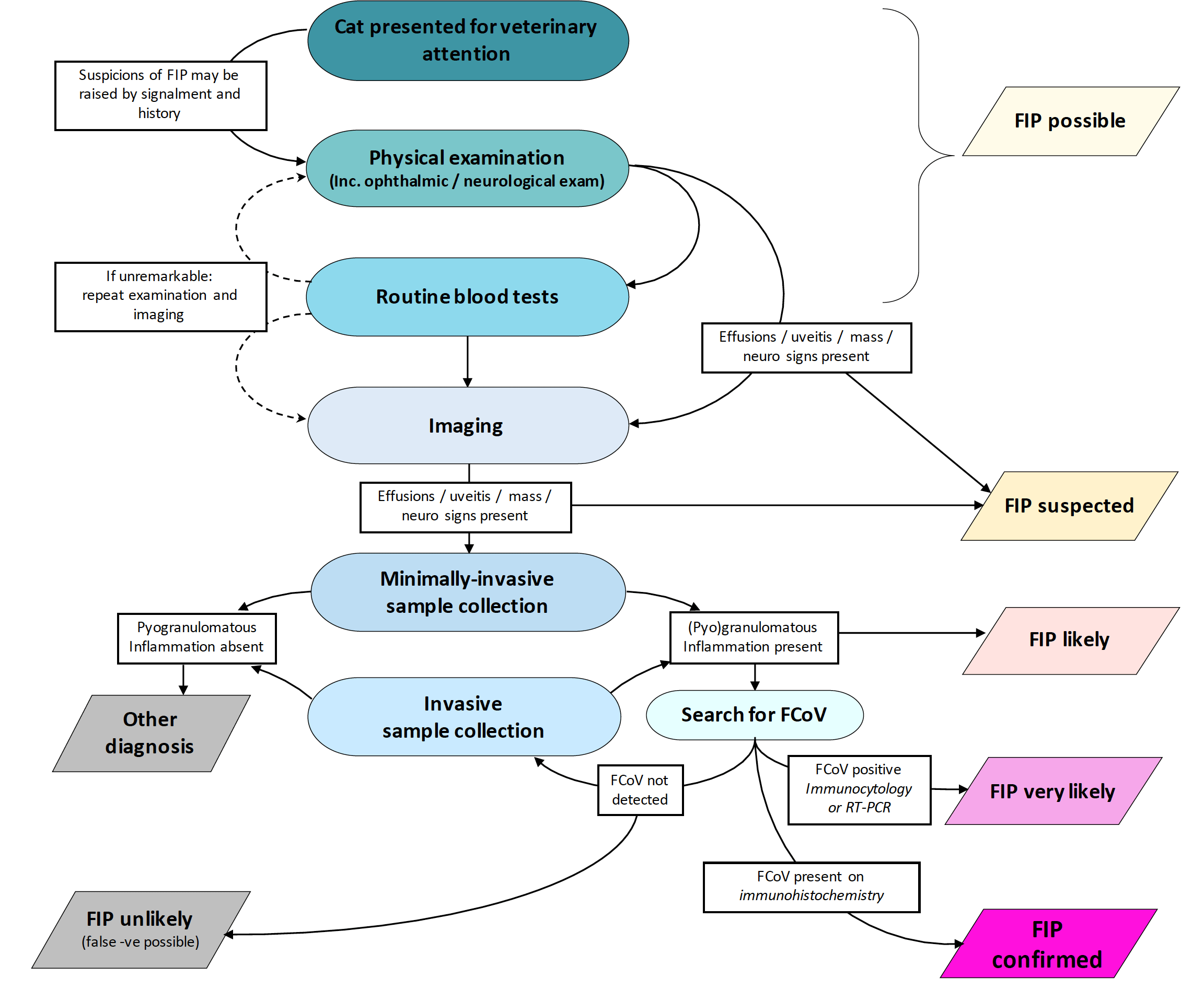
However, it is important to support a diagnosis of FIP as much as possible prior to treatment, as many other differential diagnoses exist for the clinical signs mentioned – including neoplasia (particularly lymphoma), other infectious diseases (pyothorax, toxoplasmosis, mycobacteriosis, fungal infections) and primary immune-mediated disease (idiopathic encephalitis, uveitis, lymphocytic cholangitis). See Figure 6 for a chart outlining diagnostic pathways.
Minimally invasive sample collection can include sampling of abdominal or thoracic effusions and FNAs of abnormal organs.
More detailed diagnostic flow charts for reaching a diagnosis of FIP are available via the European Advisory Board on Cat Diseases website at (www.abcdcatsvets.org/feline-infectious-peritonitis).
Over recent years, publications have focused on antiviral drugs (GS-441524, a nucleoside analogue that inhibits the viral RNA polymerase, and GC376, a viral protease inhibitor) with the potential to cure cats with experimentally induced (Kim et al, 2016; Murphy et al, 2018) and naturally acquired (Pedersen et al, 2018; 2019; Dickinson et al, 2020) FIP.
Unfortunately, until very recently (see further on), legal formulations of these medications were not commercially available, although some owners have sourced and administered illegal formulations to their cats of unknown provenance, and at great expense.
Remdesivir, a prodrug of GS-441524, is an antiviral drug with a broad-spectrum of activity against RNA viruses. It was originally developed to treat hepatitis C virus and Ebola virus in humans. Its development was then fast-tracked for the worldwide treatment of SARS-CoV-2.
In Australia, remdesivir has been legally available to vets for several months as a “special” formulation, allowing clinicians to gain experience with this drug for the treatment of cats and kittens with FIP, where it has shown great promise. Unlike GS-441524, remdesivir has low oral bioavailability and is given as an IV infusion in human patients.
In the UK, remdesivir is legally available via Gilead Sciences, which holds the patent and manufactures the product for human use. The currently available formulation is Veklury, a powder for reconstitution with water for injection to a final remdesivir concentration of 5mg/ml. Once reconstituted it should be refrigerated and used within 24 hours.
In cats, anecdotally it is generally given SC, although some cats may benefit from initial IV administration. From August 2021, remdesivir will also be available via a specials manufacturer as a veterinary “special” (Figure 7) in the UK. The reformulated remdesivir will be supplied in vials containing 100mg remdesivir, at 10mg/ml allowing for smaller injection volumes, with a shelf life when breached aseptically and stored appropriately of at least three months.

The experience of our colleagues in Australia (Malik, personal communication) allows us to make dose recommendations as outlined in the box. The authors emphasise the need for a diagnosis of FIP prior to the use of this medication to ensure it is used appropriately, while accepting that the diagnosis of FIP may be presumptive due to clinical or financial diagnostic limitations.
Costs, the prolonged nature of the treatment course (a minimum of 12 weeks is recommended), potential discomfort from SC injections and risk of relapse must be discussed with cat owners prior to commencing therapy.
Owners may be taught to administer the daily injections to their cat, but they must be carefully trained to avoid inadvertent self-injection, incorrect technique that may hurt the cat, and to minimise the risk of an adverse response from the cat in response to the injection leading to bite or scratch injuries. This treatment course requires committed owners and is an emotional as well as significant financial commitment.
Depending on the clinical condition of the cat, supportive therapy is still required (for example, IV or SC fluids, antiemetic and appetite stimulation, analgesia, nutritional support via feeding tube, antimicrobials for sepsis). Cats with uveitis may require topical therapy with corticosteroids and cats with neurological signs may need antiseizure medications.
Although systemic corticosteroid use is not generally advised alongside the use of antivirals, in cats strongly suspected of secondary immune-mediated disease as a result of the FIP (for example, immune-mediated haemolytic anaemia), a short course of corticosteroids may be considered.
Response rates are high – 80% to 95% (Malik, personal communication; Figure 8) – and, therefore, we have reason to be optimistic when discussing treatment with clients, while mindful of the commitment and costs involved, and potential for relapse.

The treatment course is long and remdesivir can be painful on injection. The drug is stored in the fridge once breached and should be brought to room temperature before injection. Needle size can influence discomfort; more rapid injection via larger bore “green” needle (21G) may be preferable for some patients, while others benefit from a smaller bore “orange” (25G) needle. A new needle should be used for each injection.
Some cats will need to come to the clinic daily for injections, and this can be done in nursing appointments. Gabapentin or trazodone (both at 50mg to 100mg orally per cat), given two hours before appointments to reduce anxiety and pain, may benefit some cats. Others may need a dose of buprenorphine (0.02mg/kg to 0.03mg/kg transmucosally or IM in clinic before treatment).
The injection site can be clipped and EMLA local anaesthetic cream applied 45 to 60 minutes prior to injection to reduce discomfort. Tolerance of injection seems to vary between individual cats.
Treatment dosages
Route of administration
Treatment duration
Monitoring
*Higher doses may be required depending on response. Using lower doses to reduce cost may increase the likelihood of treatment failure. Please note that these dosage recommendations may change according to the growing evidence base and clinical experience of clinicians using this drug. Consultation with a feline or internal medicine specialist may be advised to discuss the individual case and appropriate dose.
Researchers are investigating any beneficial effects of immunostimulants and/or other antiviral drugs, such as interferons or mefloquine, once the course of remdesivir has been completed or if injections are deemed too painful. Most published studies to date have focused on the use of the antivirals alone.
The authors wish to thank Australian colleagues David Hughes, Rebecca Brady and Richard Malik for sharing their experiences of remdesivir treatment in FIP cats. Séverine Tasker and Prof Gunn-Moore are also thanked for contributions to the article.
If advice is needed on the diagnosis and treatment of a suspected case of FIP, email [email protected]
