8 Apr 2019
Gastrointestinal disease: new technologies aiding diagnosis
Kerry Peak and David Walker summarise clinical applications of some more novel diagnostic tests for these concerns in small animals.

Gastrointestinal (GI) signs are frequently encountered in small animal primary care practice, representing just less than 25% of presenting complaints to veterinary surgeons1. A thorough understanding of the diagnosis of acute and chronic GI disease, including routinely available tests and newer developments, is essential.
A thorough history may be useful in helping determine if clinical signs are due to a primary GI problem or the result of a secondary, extra-GI condition. Determining an accurate time frame of the clinical signs is also important, as acute and chronic conditions generally require a different diagnostic and management approach.
Physical examination may demonstrate dehydration or hypovolaemia, which can indicate severity. Dehydration indicates loss of fluid from the intracellular and extracellular spaces, and can be recognised by the presence of dry mucous membranes, a skin tent or sunken eyes. Hypovolaemia is a result of loss of fluid from the vascular space in an acute time frame, and manifests as tachycardia and hypotension.
More focused evaluation of the GI tract should involve oral examination to check for halitosis and ulceration, which could indicate uraemia, previous toxin ingestion or potentially foreign material within the GI tract.
Abdominal palpation may demonstrate pain or structural disease, such as a foreign body, mass lesions or intussusception. Rectal examination should always be performed as it can help identify melaena, haematochezia or prostatic and anal sac problems that may cause tenesmus, as well as other structural abnormalities.
The information obtained from the history and physical examination will be used to help guide the clinician with regards to whether further investigation is required, and which tests may be helpful to try to determine a definitive diagnosis.
The range of more traditional diagnostic tests used to investigate GI disease in dogs and cats is listed in Panel 12-5. Clinicians are likely to be familiar with their use; therefore, they are only briefly summarised here.
The remainder of this article will focus on the clinical application of more novel diagnostics in veterinary gastroenterology.
Advanced imaging
Most clinicians are widely familiar with the use of radiographs and ultrasound; however, CT is now being used more frequently as an imaging modality for GI disease.
While general anaesthesia has historically been required to restrict patient movement, the advent of helical scanners has reduced imaging time and allowed sedation to replace anaesthesia in some situations.
A study comparing CT and abdominal ultrasound in dogs greater than 25kg showed more abdominal lesions were detected overall with CT6.
Additionally, non-contrast CT has been shown to be more accurate than ultrasound for detecting mechanical obstruction in dogs, with a positive predictive value of 100% compared with 93% using ultrasound7.
Another study showed CT was more likely to accurately diagnose acute pancreatitis and associated complications than ultrasound – especially portal vein thrombosis8. However, regardless of bodyweight, ultrasound was found to be more sensitive for identifying pathology within the bowel (wall thickening, mucosal speckles).
With the increasing availability of CT to first opinion practitioners, this is likely to become a more widely used imaging modality over the coming years.
Biomarkers
Increasing interest has occurred in the development and application of biomarkers in GI disease – especially surrounding their use in canine chronic inflammatory enteropathies. Biomarkers are generally popular among clinicians due to their ease of use.
In general terms, for a biomarker to be clinically useful, it must do some combination of the following9:
- be helpful in evaluating organ function
- assess the risk for disease development
- diagnose a specific disease process
- assess disease activity or severity
- predict the patient’s response to treatment
- predict disease outcome
- monitor disease progress
C-reactive protein
C-reactive protein (CRP) is now readily available to primary care practitioners – especially given the more recent ability for use with in-house analysers. CRP is an acute-phase protein and non-specific marker of inflammation.
On the basis of a study, it may have some use in decision-making in dogs with chronic enteropathies.
Serum CRP concentration greater than or equal to 9.1mg/L distinguished dogs with chronic enteropathy requiring anti-inflammatory or immunosuppressive treatment from those dogs responding to an elimination diet or antibiotic trial with high sensitivity (72%) and specificity (100%)10.
Methylmalonic acid
Clinicians are usually familiar with the use of cobalamin in relation to patients with suspected chronic enteropathies. Increased production of methylmalonic acid (MMA) is a marker for cobalamin deficiency at a cellular level.
Determining both serum cobalamin and serum or urine MMA may be superior to just serum cobalamin, and while this is not routinely evaluated in companion animals due to the cost and technical difficulty, it may become a future strategy9.
Faecal α1-proteinase inhibitor
Faecal α1-proteinase inhibitor (PI) is a proteolysis-resistant protein similar in size to albumin, which, with protein-losing enteropathy (PLE), should be lost into the GI tract at approximately the same rate.
Increased faecal concentrations are a marker for GI protein loss, and it can be useful for early detection of disease as it can be increased before the onset of hypoalbuminaemia or clinical signs11.
The serum-to-faecal α1PI ratio was shown to improve the diagnostic accuracy for PLE in hypoalbuminaemic dogs in one study12. Additionally, this test can be especially valuable in patients with intestinal disease that have concurrent renal or hepatic disease. In these patients, measurement of faecal α1PI can help assess which portion, if any, of the protein loss can be attributed to the intestine.
Perinuclear anti-neutrophilic cytoplasmic antibodies
Perinuclear anti-neutrophilic cytoplasmic antibodies (pANCAs) are serum autoantibodies against neutrophil granule components (and suspected cross-reactivity with a GI bacterial antigen) that can be detected by indirect immunofluorescence assays.
Positivity for pANCA is associated with PLE, protein-losing nephropathy or both in soft-coated wheaten terriers, with pANCA positivity being detected more than two years before the onset of hypoalbuminaemia13.
Although pANCA is a difficult to assay, it may be useful for early detection of protein-losing disease in soft-coated wheaten terriers (where faecal α1PI also may predict GI protein loss before the development of hypoalbuminemia).
Canine calprotectin
Calprotectin (CP) comprises the soluble protein content of neutrophils and is secreted by inflammatory tissue. Faecal CP is among the most used and reliable faecal markers for inflammatory bowel disease (IBD) in humans, where concentrations have been correlated to disease activity, endoscopic findings and the degree of histological inflammation.
A study found positive correlation between faecal CP concentrations in dogs and severity of disease before treatment. Additionally, a trend for a positive correlation existed between faecal CP and histopathological scores14. It is possible this may become more clinically relevant in the future as further studies are conducted to evaluate its usefulness.
The aforementioned biomarkers are also frequently interpreted in conjunction with the canine IBD activity index (CIBDAI). While not strictly a diagnostic “test”, its inclusion in initial diagnosis and ongoing monitoring of patients should become routine for primary care practitioners.
The CIBDAI15 is a scoring system that takes into consideration five signs of GI disease (appetite, vomiting, faecal consistency, faecal frequency and weight loss) and the overall activity level of the dog. Each sign is scored 0 to 3 based on the magnitude of its alteration. These scores are then summed, yielding a total cumulative CIBDAI score that reflects clinically irrelevant disease, or the presence of mild, moderate or severe IBD.
All dogs should be scored and then reassigned a CIBDAI score after treatment. Full clinical remission can be defined as 75% or greater reduction in CIBDAI score as compared with baseline score at diagnosis.
Partial clinical remission is defined as greater than 25% to less than 75% reduction in CIBDAI score as compared with baseline value. This scoring system can provide practitioners with a more objective way to assess response to treatment in cases of canine chronic enteropathy.
GI histopathology and adjunctive tests
A detailed discussion surrounding the indications for obtaining GI biopsies (via endoscope or surgically) is beyond the scope of this article. However, once GI biopsies have been procured, clinicians should ensure their pathology laboratory is reading the biopsies according to the guidelines produced by the WSAVA GI Standardization Group16.
This will more accurately allow clinicians to gauge the confidence placed in histological interpretation. Universal usage of such a system enhances the ability of primary care clinicians and specialists to meaningfully consult on cases and critically evaluate studies.
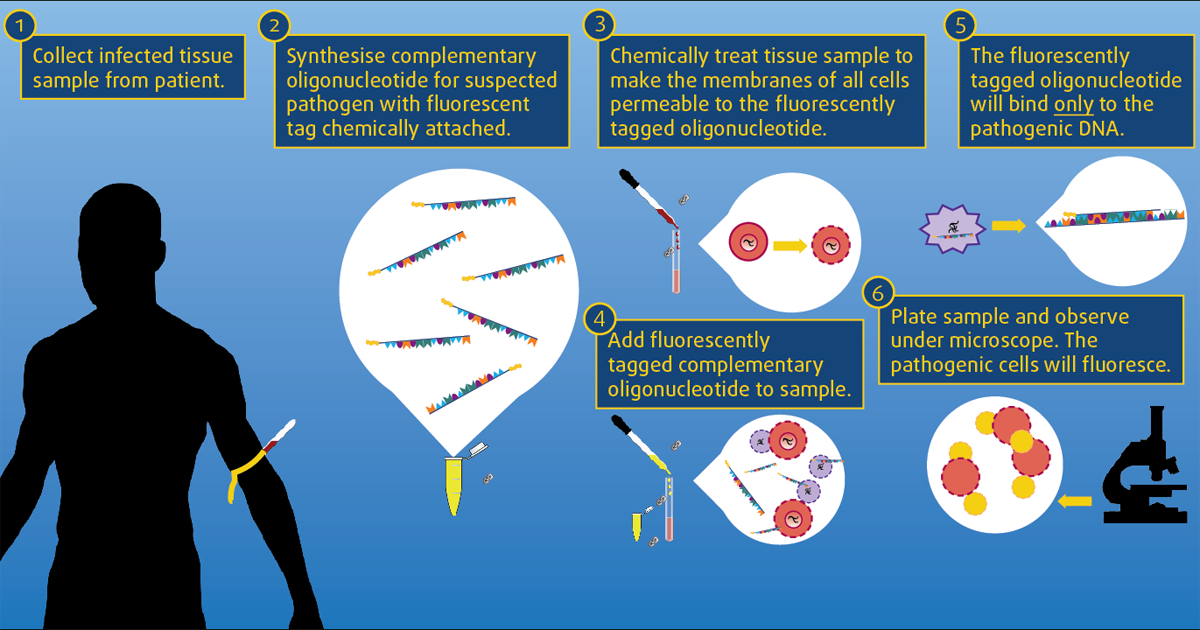
Fluorescent in situ hybridisation
Fluorescent in situ hybridisation (FISH) is another diagnostic test becoming more readily available to practitioners. It is a culture-independent method used to detect bacteria within tissues.
Fluorescent-labelled recombinant DNA probes are used to visualise bacteria and their distribution in tissue via fluorescent microscopy. The test is usually comparable in cost to routine histopathology, and can be performed on any formalin-fixed, paraffin-embedded tissue. Probes either detect most bacteria (eubacteria) or can be species specific to allow more accurate identification. These specific probes need to be requested by clinicians and, importantly, they do not give any information on antimicrobial sensitivities17,18.
FISH has enabled identification of adherent and invasive Escherichia coli as a cause of histiocytic ulcerative colitis19. FISH can also be used to demonstrate the presence of Helicobacter in cases where other diagnostic tests have been equivocal18 and this organism is considered to be clinically significant.
Campylobacter coli have also been demonstrated in cats with IBD and neutrophilic inflammation, therefore providing another potential treatment option for these patients20.
IBD or lymphoma?
Differentiating inflammation and lymphoma has sometimes been a diagnostic challenge when using traditional histopathology. Immunophenotyping has become an important diagnostic tool in differentiating between CE and intestinal lymphoma when histological changes are ambiguous; and, more recently, immunohistochemistry (IHC) and PCR for antigen receptor rearrangement (PARR) are being more frequently employed by clinicians (often when suggested by pathologists). IHC detects lineage markers and, therefore, whether a clonal expansion or mixed population of lymphocytes exists.
PARR is a PCR test that amplifies the variable regions of immunoglobulin genes and T-cell receptor genes to detect the presence of a clonal population. It, therefore, determines if lymphocytes are clonal (neoplastic) or inflammatory. To aid in differentiating inflammation from lymphoma, these two modalities can be applied.
Both procedures can be performed on paraffin wax-embedded tissue; therefore, the clinician needs only to collect a single set of biopsies for all diagnostic purposes. While clinically useful, PARR is not a perfect technique and the results should be interpreted with caution. It is neither 100% sensitive or specific – meaning that both false-negatives and false-positives can occur.
In a study that analysed endoscopically obtained biopsies from 12 canine GI lymphoma cases, PARR had a sensitivity of 66.7%21. In contrast, PARR of full-thickness intestinal samples detected monoclonal rearrangements in 76% of canine lymphoma cases, and polyclonal patterns in 100% of enteritis and normal cases22.
The tumour proliferation rate is widely estimated immunohistochemically using the Ki67 antibody MIB-1. The Ki67 protein is a cellular marker for proliferation and its level of expression has been used as a prognostic determination index in a number of neoplasms.
A study examining canine endoscopic biopsies evaluated Ki67 index, IHC and PARR. Ki67 index was greater in dogs with lymphoma than in dogs with IBD23, and this may be helpful in combination with both IHC and PARR. In feline lymphoma, PARR is less sensitive for diagnosing lymphoma than in dogs and IHC is usually superior24. Therefore, a combination of techniques will increase the likelihood of achieving a correct diagnosis25.
The interpretation of clonality tests requires integration of clinical, morphological and immunophenotypical data. These molecular studies should, therefore, be performed as an adjunct rather than replacing routine histopathological examination, and it is suggested results of immunohistochemical studies should take precedence over clonality assay in the determination of lymphocyte lineage24.
Dysbiosis index
Increasing interest has occurred in the GI microbiome over the past decade. The GI microbiome consists of several hundred bacterial species, comprising 1010 to 1014 microbial cells, and it plays a role in enterocyte health, digestion, prevention of enteropathogenic infections, and immune system regulation26.
An abnormal microbiome is thought to be a major contributing factor to immune system dysregulation, and dysbiosis (an altered composition of the bacterial microbiota) is linked to chronic enteropathies in people and animals. Dysbiosis may be a cause or effect of several GI conditions. The dysbiosis index (DI) is a rapid, quantitative PCR-based assay that quantifies eight groups of bacteria in faeces (total bacteria, Faecalibacterium species, Turicibacter species, Streptococcus species, E coli, Blautia species, Fusobacterium species and Clostridium hiranonis) and reflects microbiota changes as a single value.
It is a means of evaluating the microbiome (as most of the bacteria are not cultivatable with routine culture); a negative DI indicates normal faecal microbiota; a positive DI indicates dysbiosis; and the higher the DI, the more abnormal the microbiota.
A DI of 0 has a sensitivity of 84% and a specificity of 86% for differentiating between dysbiosis and normobiosis in dogs27. Indications for evaluating DI may include chronic enteropathies, exocrine pancreatic insufficiency, antibiotic-induced diarrhoea and pre-faecal/post-faecal transplantation.
Enough evidence is available to link dysbiosis with several different enteropathies, and evaluation of the microbiota has led to important discoveries in human gastroenterology. At this time, specific treatment recommendations cannot be made for the different types of dysbiosis, but future studies will likely guide our therapeutic strategies.
Advances in endoscopy
Summary
The range of diagnostic tests available to practitioners investigating GI disease is increasing. This article has aimed to provide readers with a summary of the current or potential clinical applications of some of the more novel diagnostic tests available.
