22 Jun 2022
Gastrointestinal disorders of cats and dogs: latest innovations
Luis Miguel García Roldán, Robert Weekes and Gerard Olivares discuss some of the latest findings for diagnosing and treating these common conditions in cats and dogs.

Image: © mrstam / Adobe Stock
Gastrointestinal disorders are among the most common presenting complaints in small animal veterinary practice.
The initial diagnostic approach to the patient with a gastrointestinal disorder should include evaluation of signalment, history and physical examination.
The clinician needs to establish the timeframe of clinical signs (acute, less than three weeks, or chronic, more than or equal to three weeks); severity; anatomical localisation (upper and/or lower gastrointestinal disease, extra-intestinal disease); and identify “alarm” signs that may warrant more urgent action.
This will help a list of differential diagnoses to be formulated and the most pertinent diagnostics to be chosen for each patient.
The aim of this article is to compile the relevant advances in diagnosis and management of gastrointestinal disorders, including the use of biomarkers, infectious disease testing, diagnosis of low-grade intestinal lymphoma and alteration of the intestinal microbiome.
Chronic inflammatory enteropathies and biomarkers
Chronic inflammatory enteropathies (CIE) are characterised by chronic (more than three weeks) or recurrent gastrointestinal signs, histologic evidence of mucosal inflammation, and the exclusion of other underlying gastrointestinal or extra-intestinal diseases (Washabau et al, 2010).
CIE are defined by their response to empirical treatment, including food-responsive enteropathy, antibiotic responsive enteropathy, immunosuppressant responsive enteropathy and non-responsive enteropathy (Dandrieux, 2016).
Additionally, patients with loss of protein across the gastrointestinal tract are grouped as protein-losing enteropathy (PLE; Dandrieux, 2016).
PLE can also be caused by primary lymphangiectasia, lymphoma or infectious diseases, among others.
While CIE is common in small animal patients, the definitive diagnosis ultimately relies on extensive work-up, including intestinal biopsies or lengthy dietary trials.
Therefore, a special interest exists in developing biomarkers in patients with CIE that can aid in diagnosing, monitoring or predicting which treatment modality will benefit each patient. Several biomarkers have been evaluated in dogs with CIE. However, few are used in the routine clinical setting and further studies are needed to critically evaluate their utility.
Cobalamin (vitamin B12)
Mucosal disease affecting the ileum reduces the epithelial expression and/or function of the B12 receptor, leading to a reduced uptake of cobalamin (vitamin B12; Kather et al, 2020).
Incidence of hypocobalaminaemia in dogs with CIE ranges from 19% to 38% (Berghoff et al, 2013), with a similar incidence in cats (Simpson et al, 2001).
Hypocobalaminaemia has been reported as a negative prognostic factor for dogs with CIE (Berghoff et al, 2013), and these patients require parenteral or oral supplementation (Kather et al, 2020). Other causes of hypocobalaminaemia include exocrine pancreatic insufficiency or hereditary disorders, the latter described in breeds such as the beagle, border collie, giant schnauzer and Australian shepherd dog (Kather et al, 2020).
While the serum concentration of B12 is commonly used diagnostically, this does not directly reflect the state of B12 at the cellular level.
Most reactions catalysed by cobalamin occur in the mitochondria, and a lack of intracellular B12 leads to reduced enzyme activity with accumulation of methylmalonic acid (MMA) and homocysteine (HCY; Kather et al, 2020). Assays for MMA and HCY are not routinely available, although both are considered as useful markers to determine the cellular cobalamin status of dogs and cats (Kather et al, 2020).
Folate (vitamin B9)
Hypofolataemia can result from chronic malabsorption in the proximal small intestine. However, the benefit of supplementing folate in dogs and cats with hypofolataemia has not been clearly demonstrated (Texas A&M University; TAMU, 2022).
Small intestinal dysbiosis may result in an increased ability of the intestinal microbiota to synthesise folate, which can lead to hyperfolataemia.Hyperfolataemia is not predictive of a positive response to antibiotics (TAMU, 2022).
Faecal alpha1-proteinase inhibitor (α1-PI)
Canine alpha1-proteinase inhibitor (α1-PI) has a molecular weight similar to albumin, and with diseases causing gastrointestinal protein loss, both are lost at a similar rate. Unlike albumin, α1-PI resists proteolysis, allowing its extraction and quantification in faecal samples (Heilmann et al, 2018a).
Increased faecal α1-PI concentration has been shown to reflect gastrointestinal protein loss and histologic lesions seen with PLE in dogs (Heilmann et al, 2016). To the authors’ knowledge, this test is not available in the UK.
Promising biomarkers
Biomarkers such as faecal calprotectin, calgranulin and perinuclear anti-neutrophilic cytoplasmic antibodies have shown promising results in differentiating sub-groups of CIE and predicting outcome.
Further research is needed to evaluate the utility of these and other biomarkers in clinical practice (Heilmann et al, 2018b).
Fluorescence in situ hybridisation
Fluorescence in situ hybridisation (FISH) can detect intact bacteria, protozoa and algae within tissues, and is readily available in the UK.
This technique has been vital in the development of veterinary gastroenterology, including the identification of attaching and invasive Escherichia coli causing granulomatous colitis in the boxer dog (Simpson et al, 2006).
FISH can be requested in addition to conventional histology when the presence of enteropathogens is suspected and when overt neutrophilic or histiocytic inflammation is present.
Interestingly, two studies in feline intestinal biopsies with neutrophilic inflammation identified infections with Tritrichomonas foetus and Campylobacter coli, in colonic and duodenal biopsies, respectively (Yaeger and Gookin, 2005; Maunder et al, 2016).
The clinician needs to be careful when interpreting FISH results, because the presence of a positive result may represent a primary infection, but also secondary overgrowth or disruption of the mucosal barrier.
Low-grade intestinal T-cell lymphoma in cats
Intestinal small cell lymphoma (SCL) in cats is the most frequent digestive tract neoplasia, and histology consists of small, well-differentiated lymphocytes with low mitotic rates (Paulin et al, 2018). More than 90% of intestinal SCL cases are of T-cell origin and are currently referred to as low-grade intestinal T-cell lymphoma (LGITL).
In practice, differentiating between lymphoplasmacytic enteritis (LPE) and LGITL remains challenging. A recent study revealed some clinical and ultrasonographic variables that can guide clinicians (Freiche et al, 2021a; Table 1), but, ultimately, intestinal biopsies are required to achieve a definitive diagnosis.
| Table 1. Signalment, clinical data and ultrasonographic findings of cats diagnosed with low-grade intestinal T-cell lymphoma (LGITL) or lymphoplasmacytic enteritis (LPE). Adapted from Freiche et al, 2021 | ||
|---|---|---|
| Variable | LGTIL | LPE< |
| Male sex* | 16/22 (73 per cent) | 6/22 (27 per cent) |
| Weight loss | 13/22 (59 per cent) | 13/22 (56 per cent) |
| Vomiting | 15/22 (68 per cent) | 18/22 (82 per cent) |
| Polyphagia (%) | 6/22 (27 per cent) | 1/22 (5 per cent) |
| Duration of clinical signs (median; range)* | 365 (62-1,460) | 107 (7-1,095) |
| Small intestinal diarrhoea* | 14/22 (64 per cent) | 6/22 (27 per cent) |
| Hypocobalaminaemia (B12 less than 200 nanograms/litre)* | 12/21 (57 per cent) | 4/21 (19 per cent) |
| Abdominal effusion* | 10/22 (45 per cent) | 3/22 (14 per cent) |
| Ultrasound of jejunal lymph nodes | ||
| Rounded shape* | 17/20 (85 per cent) | 1/17 (6 per cent) |
| Hypoechoic* | 13/20 (65 per cent) | 2/17 (12 per cent) |
| Increased perinodal fat echogenicity* | 14/20 (70 per cent) | 3/17 (18 per cent) |
| *Parameters found to be significantly different between groups (p-value less than 0.05). | ||
Biopsies can be obtained via endoscopy or exploratory laparotomy. Historically, the latter was considered superior to differentiate between LPE and LGITL; however, less invasive endoscopic biopsies with ancillary testing can be sufficient in some cases.
The main limitation to endoscopic biopsies is the localisation of LGITL, since 64% of cases have infiltration of the jejunum only, which cannot be reached endoscopically (Freiche et al, 2021b).
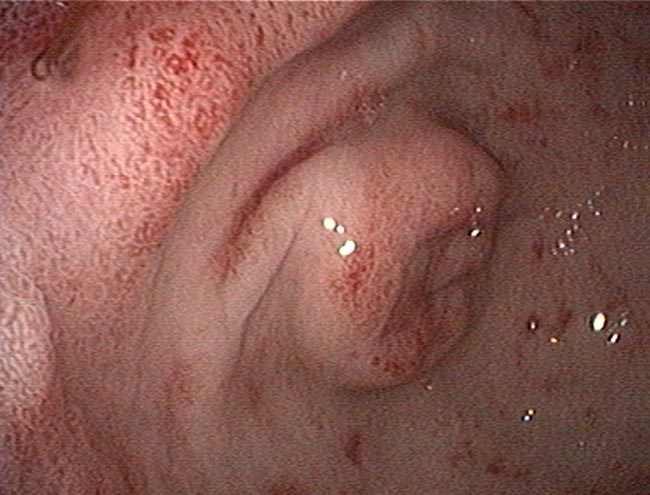
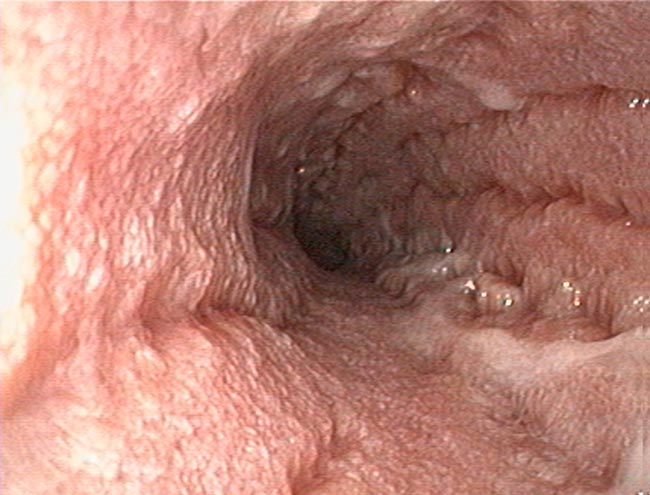
Immunohistochemistry
Immunohistochemistry (IHC) involves the use of antibodies against specific antigens in tissue sections.
When lymphoma is suspected, a panel of T- and B-cell antibodies (for example, CD3 and CD20) can identify a monomorphic population, and establish the cell lineage. Antibodies such as Ki67, pSTAT3 and pSTAT5 have been recently studied, and are useful for the diagnosis of LGTIL (Freiche et al, 2021b). The combination of histopathology and IHC is considered the gold standard for diagnosis of LGTIL.
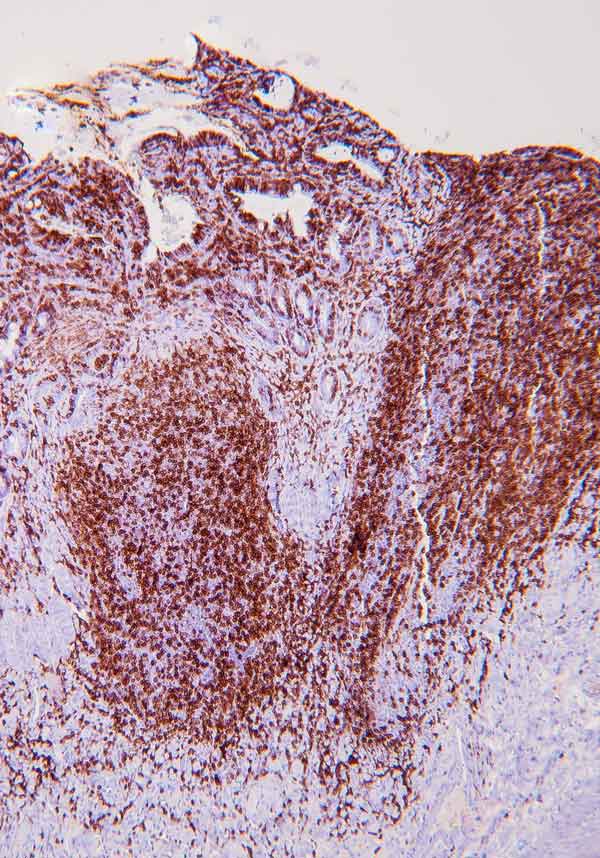
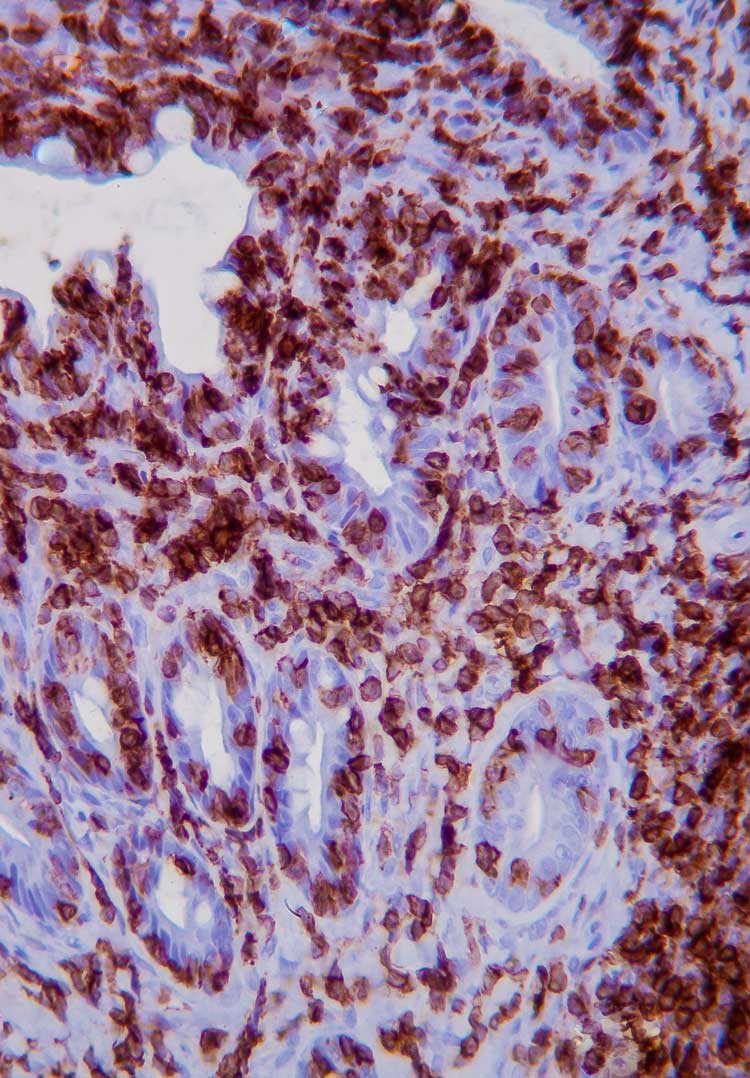
PCR for antigen receptor rearrangement
PCR for antigen receptor rearrangement (PARR) is a clonality assay that uses primers sets for amplification of rearranged immunoglobulin heavy-chain genes, and T-cell receptor gamma for B-cell and T-cell, respectively.
Against what was initially thought, benign clonal expansions have been described in various inflammatory diseases, including LPE in cats, where 40% of cases show monoclonality (Freiche et al, 2021b).
Clinicians should be cautious about reclassifying doubtful lymphoma based on PARR results.
Intestinal microbiome
Abnormalities in gut microbial populations have been associated with a variety of gastrointestinal and systemic diseases, making the intestinal microbiome a viable diagnostic and therapeutic target. Advances in DNA sequencing and computational technology are increasing our understanding of this field, but many fundamental questions remain unanswered (Barko et al, 2018).
Studies have identified decreased bacterial diversity in both CIE and acute gastroenteritis (Suchodolski et al, 2012), and have shown increased populations of potentially harmful species such as Proteobacteria and Clostridium perfringens (Ziese et al, 2018).
However, a lack of clarity exists about whether these changes are the cause or consequence of the disease process.
The dysbiosis index (DI) is a faecal PCR-based diagnostic test that gives a quantitative measure of the presence of bacterial groups, counts total bacterial numbers and summarises them in one single number (TAMU 2022). This test is now available commercially in some UK laboratories.
While a growing body of evidence exists supporting DI as a therapeutic target for cases receiving probiotics and faecal microbiota transplant (FMT; TAMU, 2022), further research is needed to define the clinical interpretation of DI.
Probiotics
Probiotics are formulations of live organisms that confer beneficial effects on the recipient when delivered in adequate amounts.
A detailed guide of the use of probiotics in dogs and cats is beyond the scope of this article and further information can be found in the literature (Barko et al, 2018; Jugan et al, 2017).
The authors have highlighted some examples of the potential benefits of probiotics in dogs and cats with gastrointestinal disease:
Canine parvovirus: In one small controlled study, the use of probiotic bacteria (VSL#3) reduced clinical signs, increased leukocyte counts and improved survival rates, compared with control dogs (Arslan et al, 2012). However, this study had several limitations, such as the lack of faecal microbiome evaluations, and the method of randomisation was not described.
Tritrichomonas foetus in cats: Evidence exists supporting probiotic use (Enterococcus faecium SF68 or isolated strain of Enterococcus hirae), alongside ronidazole in cats with Tritrichomonas foetus infections. A study found significant improvements in faecal scoring and bodyweight, as well as a reduced relapse rate (Lalor and Gunn-Moore, 2012).
CIE: VSL#3 showed a protective effect in dogs with CIE by decreasing clinical and histologic scores when compared with a control group that was treated with metronidazole and prednisolone (Rossi et al, 2014).
Further research is needed to fully understand the underlying mechanisms by which probiotics are effective in a range of enteropathies, so their use can be guided by an evidence-based approach.
Antibiotics
In the past, antibiotics were widely used for acute and chronic gastrointestinal disease. However, this is now debated, given the age of increasing antibiotic resistance and the prolonged intestinal dysbiosis seen in healthy dogs and humans (Grønvold et al, 2010).
Moreover, strong evidence exists that antibiotic use is not required in uncomplicated cases affected by enteropathogenic bacteria and acute haemorrhagic diarrhoea syndrome (AHDS; Marks et al, 2011; Werner et al, 2020).
When dealing with suspected or confirmed infectious enteropathogens, especially those with zoonotic risk (for example, Salmonella and Campylobacter), supportive treatment, use of appropriate protective equipment, and proper cleaning and disinfection are the mainstays of control (Marks et al, 2011).
The majority of dogs hospitalised with AHDS improve rapidly with symptomatic treatment only, so the use of antibiotics is not routinely recommended.
A majority of patients affected by mild to moderate CIE are known to respond to diet alone (food-responsive enteropathy), so the recommendation is to attempt dietary management in the first instance (Dandrieux, 2016).
In recent years, many clinicians have moved away from using antibiotics for CIE, and these are being reserved for those patients with history of positive antibiotic response and refractory to other treatments.
Faecal microbiota transplantation
Faecal microbiota transplantation (FMT) involves introducing faeces from a healthy donor into the gut of a diseased recipient, with the aim to modulate or replace the recipient’s intestinal microbiota (Schmitz, 2022).
FMT is used in human medicine for the treatment of Clostridium difficile infections, and has been shown to stabilise and increase the diversity of the microbiome (Kao et al, 2017). Early studies of this effect in veterinary medicine are promising and suggestive of a similar outcome (Sugita et al, 2019).
A single FMT administered to dogs with AHDS significantly increased the diversity of the microbiome and the population of short-chain fatty acid – producing bacterial strains in comparison to a control group (Gal et al, 2021).
However, no improvement was observed in clinical outcomes post-FMT and the diversity index was not significantly different 30 days post-transplant (Gal et al, 2021).
In puppies with parvovirus infection, FMT did not significantly improve survival, but increased the percentage of survivors who had resolution of diarrhoea within 48 hours and shortened hospitalisation times (Pereira et al, 2018).
FMT is being increasingly used in refractory cases of CIE; however, a need exists for prospective controlled studies to fully quantify the benefits of FMT, as well as consensus regarding preparation, dosing and administration (Schmitz, 2022).
The interested reader is referred to the current review on FMT (Chaitman and Gaschen, 2021).
Another area of growing interest is the analysis of metabolomics in dogs and cats with gastrointestinal disease. For instance, results in a study of dogs with acute diarrhoea indicated that the faecal dysbiosis was associated with significant changes in profiles of serum and urine metabolites (Guard et al, 2015).
As with FMT, more research is needed to understand the importance and utility of metabolite analysis.
- Some of the drugs mentioned in this article are used under the cascade.
References
- Arslan HHA, Aksu D, Terzi G and Nisbet C (2012). Therapeutic effects of probiotic bacteria in parvoviral enteritis in dogs, Revue Med Vet 163(2): 55-59.
- Barko PC, McMichael MA, Swanson KS and Williams DA (2018). The gastrointestinal microbiome: a review, J Vet Intern Med 32(1): 9-25.
- Berghoff N, Parnell NK, Hill SL et al (2013). Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease, Am J Vet Res 74(1): 84-89.
- Berghoff N and Steiner JM (2011). Laboratory tests for the diagnosis and management of chronic canine and feline enteropathies, Vet Clin North Am Small Anim Pract 41(2): 311-328.
- Chaitman J and Gaschen F (2021). Fecal microbiota transplantation in dogs, Vet Clin North Am Small Anim Pract 51(1): 219-233
- Dandrieux J (2016). Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract 57(11): 589-599
- Freiche V, Fages J, Paulin MV et al (2021a). Clinical, laboratory and ultrasonographic findings differentiating low-grade intestinal T-cell lymphoma from lymphoplasmacytic enteritis in cats, J Vet Intern Med 35(6): 2,685-2,696.
- Freiche V, Paulin MV, Cordonnier N et al (2021b). Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis, J Vet Intern Med 35(6): 2,673-2,684.
- Gal A, Barko PC, Biggs PJ et al (2021). One dog’s waste is another dog’s wealth: a pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome, PLoS One 16(4): e0250344.
- Grønvold AM, L’Abée-Lund TM, Sørum H et al (2010). Changes in fecal microbiota of healthy dogs administered amoxicillin, FEMS Microbiol Ecol 71(2): 313-326.
- Guard BC, Barr JW, Reddivari L et al (2015). Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea, PLoS One 10(5): e0127259.
- Heilmann RM, Parnell NK, Grützner N et al (2016). Serum and fecal canine α1-proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs, Vet J 207: 131-139.
- Heilmann RM and Steiner JM (2018a). Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs, J Vet Intern Med 32(5): 1,495-1,508.
- Heilmann RM, Berghoff N, Mansell J et al (2018b). Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies, J Vet Intern Med 32(2): 679-692.
- Jugan MC, Rudinsky AJ, Parker VJ and Gilor C (2017). Use of probiotics in small animal veterinary medicine, J Am Vet Med Assoc 250(5): 519-528.
- Kao D, Roach B, Silva M et al (2017). Effect of oral capsule vs colonoscopy delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial, JAMA 318(20): 1,985-1,993.
- Kather S, Grützner N, Kook PH et al (2020). Review of cobalamin status and disorders of cobalamin metabolism in dogs, J Vet Intern Med 34(1): 13-28.
- Lalor SM and Gunn-Moore DA (2012). Effects of concurrent ronidazole and probiotic therapy in cats with Tritrichomonas foetus-associated diarrhoea, J Feline Med Surg 14: 650-658.
- Marks SL, Rankin SC, Byrne BA and Weese JS (2011). Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control, J Vet Intern Med 25(6): 1,195-1,208.
- Maunder C, Reynolds Z, Peacock L et al (2016). Campylobacter species and neutrophilic inflammatory bowel disease in cats, J Vet Intern Med 30(4): 996-1,001.
- Paulin MV, Couronné L, Beguin J et al (2018). Feline low-grade alimentary lymphoma: an emerging entity and a potential animal model for human disease, BMC Vet Res 14(1): 306.
- Pereira GQ, Gomes LA, Santos IS et al (2018). Fecal microbiota transplantation in puppies with canine parvovirus infection, J Vet Intern Med 32(2): 707-711.
- Rossi G, Pengo G, Caldin M et al (2014). Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease, PLoS One 9(4): e94699.
- Schmitz SS (2022). Observational study of small animal practitioners’ awareness, clinical practice and experience with fecal microbiota transplantation in dogs, Top Companion Anim Med 47: 100630.
- Simpson KW, Fyfe J, Cornetta A (2001). Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease, J Vet Intern Med 15(1): 26-32.
- Simpson KW, Dogan B, Rishniw M et al (2006). Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs, Infect Immun 74(8): 4,778-4,792.
- Suchodolski JS, Markel ME, Garcia-Mazcorro JF et al (2012). The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease, PLoS One 7(12): e51907.
- Sugita K, Yanuma N, Ohno H et al (2019). Oral faecal microbiota transplantation for the treatment of Clostridium difficile-associated diarrhoea in a dog: a case report, BMC Vet Res 15(1): 11.
- Texas A&M University (TAMU) College of Veterinary Medicine and Biomedical Sciences (2022). Cobalmin information, https://vetmed.tamu.edu/gilab/research/cobalamin-information
- Washabau RJ, Day MJ, Willard MD et al (2010). Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals, J Vet Intern Med 24(1): 10-26.
- Werner M, Suchodolski JS, Straubinger RK (2020). Effect of amoxicillin-clavulanic acid on clinical scores, intestinal microbiome, and amoxicillin-resistant Escherichia coli in dogs with uncomplicated acute diarrhea, J Vet Intern Med 34(3): 1,166-1,176.
- Yaeger MJ and Gookin JL (2005). Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats, Vet Pathol 42(6): 797-804.
- Ziese AL, Suchodolski JS, Hartmann K et al (2018). Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea, PLoS One 13(9): e0204691.
