12 Mar 2024
Gastrointestinal problems and latest thinking in pets
Cecilia Villaverde considers dietary strategies for combating these issues in both canine and feline patients.

Image © FurryFritz / Adobe Stock
Diet plays a role in the management of a large variety of canine and feline gastrointestinal (GI) problems – both acute and chronic.
Chronic enteropathies (CEs) – also called chronic inflammatory enteropathies – are characterised by GI signs for three weeks or longer associated to intestinal inflammation of unknown cause1,2.
Clinical signs are variable and can be intermittent or recurrent; these include vomiting, diarrhoea, weight loss and inappetence. In some CE cases associated with intestinal protein loss (protein-losing enteropathy; PLE), clinical signs associated with hypoalbuminaemia can also be present, such as dyspnoea.
The pathophysiology is not completely known, but different factors are believed to play a role, including genetic predisposition, environmental factors (such as diet), abnormal immune response and altered gut microbiota1,3. These diseases are classified1 according to their response to treatment into food responsive, immunosuppressive responsive and antibiotic responsive.
Microbiome aberrations in CE are receiving a lot of interest, including their therapeutic potential. Several studies exist on dogs4,5 and cats6,7 assessing faecal microbiota, comparing patients with CE with controls that support potential dysbiosis in patients with CE, and a dysbiosis index has been developed as a clinical tool8. Dysbiosis in CE can be a cause, a consequence or both.
“Different dietary strategies exist for the patients to respond to, and identifying the best one is a process of trial and error…”
Good evidence also exists that diet can affect gut microbiota both in health and disease9. As diet is a complex matrix with many variables, its effect on both clinical remission and intestinal microbiota can be on multiple levels, and can be difficult to assess. A study in cats with CE using a hydrolysed soy-based diet7, and one in dogs using a diet based on purified amino acids4, showed that those patients that responded to diet change with clinical improvement had changes in their faecal microbiota different to the non-responders, at least in the short term.
One study on dogs with steroid-responsive disease5 did not see any improvements in faecal microbiota alterations (compared to healthy controls) after three weeks of medical treatment. Future research can potentially help elucidate which patients can benefit from specific therapies (medical, dietary or others). CE in dogs and cats appears associated with aberrations in the faecal microbiome, but this is heterogeneous, as are the changes associated with therapies.
More research is needed to elucidate the role of intestinal microbiota changes in the pathogenesis of intestinal diseases, and how useful targeted therapeutic interventions are in these cases, such as diet, but also probiotics9 or faecal microbiota transplantation10.
In addition to the effects on the microbiota, dietary management is very important in canine and feline CE. For diagnostics, all suspected CE cases should undergo one or more dietary trials to identify food-responsive CE patients11. Different dietary strategies exist for the patients to respond to, and identifying the best one is a process of trial and error.
For long-term management of all cases, developing a feeding plan will help provide nutrients and energy required by the patient in a tolerated, and bioavailable, manner.
Dietary strategies for CE
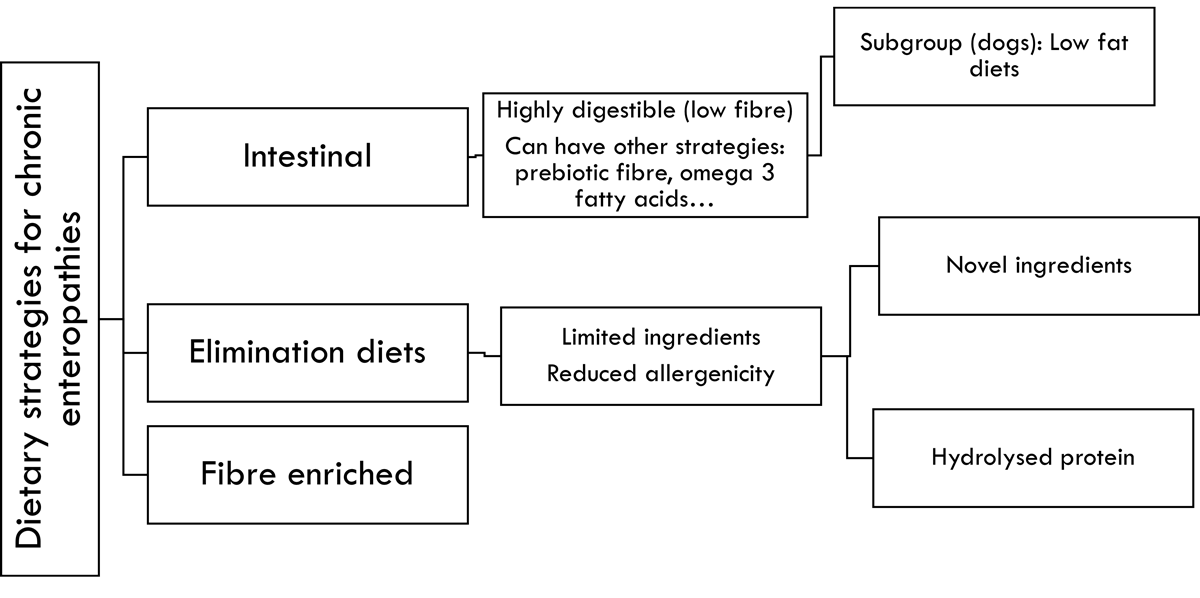
Several dietary strategies exist that can result in clinical improvement in dogs and cats with CE, although the evidence is stronger in dogs12. These are shown in Figure 1.
Highly digestible (intestinal) diets
Intestinal diets are formulated to be highly digestible, with the goal of facilitating the uptake and use of nutrients when intestinal function is compromised13. These diets can be commercially available or home prepared, and are used for a variety of intestinal disorders, acute or chronic. They often include small amounts of prebiotic fibre. Depending on the commercial product, other strategies might also be incorporated, such as omega-3 fatty acids (due to their beneficial effects in some intestinal diseases14).
The effect of these nutritional characteristics can be direct and also indirect, via their effect on the microbiota. The use of such diets has resulted in improvement in cats with suspected CE, assessed in the short term (two to six weeks)13,15,16.
Several studies exist4,17-21 supporting a positive effect of intestinal low-fat diets in canine PLE, using commercial diets with fat levels ranging between 16% and 22% on a metabolisable energy (ME) basis, or using home-made diets, which can be even lower in fat (10% to 15% ME). One of the studies18 was carried out in PLE dogs resistant to steroids that had already received a therapeutic diet using other strategies (mainly elimination diets). Therefore, in dogs with PLE, an intestinal low-fat diet – be it commercial or home-made – is a good first choice.
Elimination diets
Elimination diets are used to rule out adverse food reactions and to identify patients with classical food-responsive CE22. The reason why some CE patients respond to such a strategy suggests that a component of adverse food reaction might exist in these cases, but other modes of action (or their combination) cannot be ruled out.
These diets tend to be highly digestible and include omega-3 fatty acids, prebiotic fibre and so forth. An indirect effect via microbiota modulation is also possible4,7,23.
Elimination diets are limited in ingredients, highly digestible and have lower allergenic potential, either by using uncommon ingredients (potentially novel) or by using hydrolysed protein. Reducing protein size via hydrolysis24 can decrease its allergenicity.
Using a diet based on purified amino acids would follow the same principle. Carbohydrate sources are either uncommon, rice or purified starch (where protein has been removed from the flour).
This is a common first strategy in dogs and cats with CE. Several studies exist supporting a positive response of dogs25-30 and cats7,31-33 to this strategy, and a positive response can usually be seen within two weeks11.
A scarcity of research exists comparing novel ingredient versus hydrolysed protein, and a retrospective canine study did not find a difference in efficacy29. Commercial uncommon ingredient diets need to be assessed for novelty via diet history, which is challenging, as it relies on owner recall.
Moreover, in Europe, ingredients can be declared as categories, making identifying exposures impossible. Hydrolysed protein diets can be used with an incomplete diet history, but can be more expensive and, anecdotally, not as palatable. Wet options are also very limited for the hydrolysed protein group.
Home-made diets can also be considered, using limited ingredients novel to the patient. These can be more expensive than therapeutic dry diets34. It is also common for pet caregivers to modify the recipe, rendering it unbalanced over time35.
Fibre-enhanced diets
Dietary fibre can have a variety of effects that result in improved GI health36,37. It is not only used in fibre-enhanced diets; most diets for intestinal problems do include some (even if in lower amounts).
Sources include ingredients such as cereals (especially whole grain); tubers; legumes; and also purified sources such as bran, beet pulp, fructooligosaccharides and psyllium husk.
Fibre is a very heterogeneous group of substances, and its effects on the intestinal tract vary depending on the type, amounts and combinations. Table 1 details some of the effects of fibre on the intestinal tract38, depending on solubility in water.
| Table 1. Some effects of dietary soluble and insoluble fibre on intestinal tract physiology | ||||
|---|---|---|---|---|
| Viscosity | Intestinal transit time | Faecal volume | Faecal moisture | |
| Soluble fibre | No change or increase | No change or slow down | No change | Increase |
| Insoluble fibre | No change | Accelerate | Increase | No change or decrease |
Fibre can also be classified according to its fermentability (that is, capacity to be used by the microbiota). Fermentable fibre can play an important role in intestinal disease for its capacity to modify microbiota composition39.
Diets specifically formulated for intestinal disease (“intestinal”) that are enhanced or enriched in fibre are relatively new (more traditional fibre-enriched diets include those for the management of diabetes and excessive weight). The use of this strategy is particularly recommended in large bowel diarrhoea, and most of the research in this area has been carried out in dogs40-43.
Supplementation with a concentrated fibre source can also be considered44; this can be very helpful if the patient needs to be on a specific diet for a comorbidity.
This strategy is a good first choice for colitis, and it is worth trying different commercial diets if the first one does not work, as they vary in fibre type and amounts.
Developing a feeding plan for intestinal disease
A nutritional assessment45 should be performed to identify any nutritional risk factors and decide on the best feeding plan for each case. A diet history form is very important to identify an ingredient exposure list, current calorie intake and any strategies already tried.
Diet choice
Initial diet choice will be affected by the specific clinical presentation. Diet is a very complex matrix; when we change the diet, we change multiple things: ingredients, macronutrient and micronutrient profile, fibre types and amounts, physical form, digestibility and so forth. As such, it is difficult to attribute the success or failure of a diet trial to one single characteristic. It might be worth trying more than one diet within the same strategy, as they will differ in other important characteristics.
Some diets in the market combine multiple strategies; for example, hydrolysed protein diets that are also low fat (16% to 18% fat on a ME basis). This is very helpful when both strategies are considered important. Fibre supplementation can also be considered with different diet choices. Other factors affecting diet choice will be species and life stage (to choose a complete diet for each case) availability, cost, palatability, comorbidities and client preferences. Quality control is critical when choosing a hydrolysed protein or novel ingredient diet.
These diets might contain undeclared antigens46-49, likely due to cross-contamination; this can be more common in non-veterinary (or over-the-counter) diets. While the clinical significance of small amounts of undeclared ingredients is unknown, it is prudent to use products that are manufactured in such a way to minimise that risk.
“In a busy clinic, it can feel almost impossible to find the time to consistently measure [systolic blood pressure] in at-risk cats. However, the vast majority of clinics now have the equipment needed…”
If supplements (probiotics or others) are used, together with an elimination diet, it is important to ensure they are not providing uncontrolled antigens, which is certainly possible50. It is worth starting with diet change first and adding the supplement two weeks later, after assessing diet response alone.
In some cases, vitamin B12 supplementation is necessary51,52, as hypocobalaminaemia can be present in intestinal disease due to malabsorption or inappetence. If a home-made diet is desired (for example, to make a very low-fat diet in PLE cases where commercial low-fat diets are not enough), consulting a veterinary nutritionist for a customised recipe is indicated. Using generic recipes is associated with nutritional imbalances53,54.
Daily allowance
The goal is to provide enough food to meet weight and body condition score (BCS) goals.
In adults, this would mean weight stability with an ideal BCS55,56, while in growing animals, we want adequate rate of growth57 and ideal BCS. A good diet history can identify current calorie intake, which can then be adjusted to achieve these goals.
If the current intake cannot be determined, one can use energy requirement formulae38,58 or follow label instructions (in turn based on formulae). Formulae can have an error of plus-minus 50%58; therefore, it is important to weigh the patient at least once a month (every two weeks, initially) to adjust the allowance in 10% intervals to achieve weight goals.
Feeding method
Most patients can be fed in a portion-controlled manner. Feeding multiple meals per day (at least two) is potentially helpful, to reduce the intestinal workload per meal.
Free feeding can be considered in thin patients with picky or inconsistent appetite, but caregivers should still measure the daily food intake to identify any changes to food intake in a timely manner. For patients not eating enough to maintain their weight, assisted feeding should be considered.
Follow-up
Repeating the nutritional assessment is important to adjust the feeding plan, including diet choice and daily allowance. Clinical indices can be used to assess response to dietary treatment27,32.
Two weeks is usually sufficient to see an effect on diet, and if no improvement is observed, a different diet with differing nutritional characteristics or strategy should be considered. If the patient does not respond to several diet strategies, more diagnostics will be required.
References
- Jergens AE and Heilmann RM (2022). Canine chronic enteropathy – current state-of-the-art and emerging concepts, Frontiers in Veterinary Science 9: 923013.
- Dandrieux JRS and Mansfield CS (2019). Chronic enteropathy in canines: prevalence, impact and management strategies, Veterinary Medicine: Research and Reports 10: 203-214.
- Marsilio S (2021). Feline chronic enteropathy, Journal of Small Animal Practice 62(6): 409-419.
- Manchester AC et al (2023). Efficacy of an elemental diet in achieving clinical remission in dogs with chronic enteropathy, Journal of Veterinary Internal Medicine 37(6): 2,322-2,333.
- Minamoto Y et al (2015). Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease, Gut Microbes 6(1): 33-47.
- Marsilio S et al (2019). Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma, Scientific Reports 9(1): 19208.
- Kathrani A et al (2022). The effect of a hydrolyzed protein diet on the fecal microbiota in cats with chronic enteropathy, Scientific Reports 12(1): 2746.
- Suchodolski JS (2022). Analysis of the gut microbiome in dogs and cats, Veterinary Clinical Pathology 50(Suppl 1): 6-17.
- Pilla R and Suchodolski JS (2021). The gut microbiome of dogs and cats, and the influence of diet, Veterinary Clinics of North America: Small Animal Practice 51(3): 605-621.
- Toresson L et al (2023). Clinical effects of faecal microbiota transplantation as adjunctive therapy in dogs with chronic enteropathies – a retrospective case series of 41 dogs, Veterinary Sciences 10(4): 271.
- Kathrani A (2021). Dietary and nutritional approaches to the management of chronic enteropathy in dogs and cats, Veterinary Clinics of North America: Small Animal Practice 51(1): 123-136.
- Makielski K et al (2019). Narrative review of therapies for chronic enteropathies in dogs and cats, Journal of Veterinary Internal Medicine 33(1): 11-22.
- Perea SC et al (2017). Evaluation of two dry commercial therapeutic diets for the management of feline chronic gastroenteropathy, Frontiers in Veterinary Science 4: 69.
- Bauer JE (2011). Therapeutic use of fish oils in companion animals, Journal of the American Veterinary Medical Association 239(11): 1,441-1,451.
- Laflamme DP et al (2012). Evaluation of canned therapeutic diets for the management of cats with naturally occurring chronic diarrhea, Journal of Feline Medicine and Surgery 14(10): 669-677.
- Laflamme DP et al (2011). Effect of diets differing in fat content on chronic diarrhea in cats, Journal of Veterinary Internal Medicine 25(2): 230-235.
- Rudinsky AJ et al (2017). Dietary management of presumptive protein-losing enteropathy in Yorkshire terriers, Journal of Small Animal Practice 58(2): 103-108.
- Wennogle SA et al (2021). Prospective evaluation of a change in dietary therapy in dogs with steroid-resistant protein-losing enteropathy, Journal of Small Animal Practice 62(9): 756-764.
- Okanishi H et al (2014). The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia, Journal of Veterinary Internal Medicine 28(3): 809-817.
- Nagata N et al (2020). Clinical characteristics of dogs with food-responsive protein-losing enteropathy, Journal of Veterinary Internal Medicine 34(2): 659-668.
- Myers M et al (2023). Prospective evaluation of low-fat diet monotherapy in dogs with presumptive protein-losing enteropathy, Journal of the American Animal Hospital Association 59(2): 72-84.
- Cave N (2012). Nutritional management of gastrointestinal diseases. In Fascetti AJ and Delaney SJ (eds), Applied Veterinary Clinical Nutrition, Wiley-Blackwell, Chichester: 175–220.
- Wang S et al (2019). Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids, Microbiome 7(1): 126.
- Cave NJ and Guilford WG (2004). A method for in vitro evaluation of protein hydrolysates for potential inclusion in veterinary diets, Research in Veterinary Science 77(3): 231-238.
- Mandigers PJJ et al (2010). A randomized, open-label, positively-controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy, Journal of Veterinary Internal Medicine 24(6): 1,350-1,357.
- Walker D et al (2013). A comprehensive pathological survey of duodenal biopsies from dogs with diet-responsive chronic enteropathy, Journal of Veterinary Internal Medicine 27(4): 862-874.
- Allenspach K et al (2007). Chronic enteropathies in dogs: evaluation of risk factors for negative outcome, Journal of Veterinary Internal Medicine 21(4): 700-708.
- Marks S et al (2002). Dietary trial using a commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease, Veterinary Therapeutics 3(2): 109-118.
- Allenspach K et al (2016). Long-term outcome in dogs with chronic enteropathies: 203 cases, Veterinary Record 178(15): 368.
- Simpson KW et al (2023). Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy, Journal of Veterinary Internal Medicine 37(6): 2,334-2,343.
- Guilford WG et al (2001). Food sensitivity in cats with chronic idiopathic gastrointestinal problems, Journal of Veterinary Internal Medicine 15(1): 7-13.
- Jergens AE et al (2010). A clinical index for disease activity in cats with chronic enteropathy, Journal of Veterinary Internal Medicine 24(5): 1,027-1,033.
- Mandigers PJJ et al (2010). Efficacy of a commercial hydrolysate diet in eight cats suffering from inflammatory bowel disease or adverse reaction to food, Tijdschrift voor Diergeneeskunde 135(18): 668-672.
- Kratzer GR et al (2022). Home-cooked diets cost more than commercially prepared dry kibble diets for dogs with chronic enteropathies, Journal of the American Veterinary Medical Association 260(S3): S53-S60.
- Johnson LN et al (2016). Evaluation of owner experiences and adherence to home-cooked diet recipes for dogs, Journal of Small Animal Practice 57(1): 23-27.
- Moreno AA et al (2022). Dietary fiber aids in the management of canine and feline gastrointestinal disease, Journal of the American Veterinary Medical Association 260(S3): S33-S45.
- Chandler M (2016). Importance of fibre in diet, Vet Times 46(35): 10-12.
- National Research Council (2006). Nutrient Requirements of Dogs and Cats, The National Academies Press, Washington DC.
- Fritsch DA et al (2022). Microbiome function underpins the efficacy of a fiber-supplemented dietary intervention in dogs with chronic large bowel diarrhea, BMC Veterinary Research 18(1): 245.
- Fritsch DA et al (2022). A prospective multicenter study of the efficacy of a fiber-supplemented dietary intervention in dogs with chronic large bowel diarrhea, BMC Veterinary Research 18(1): 244.
- Lappin MR et al (2022). Efficacy of feeding a diet containing a high concentration of mixed fiber sources for management of acute large bowel diarrhea in dogs in shelters, Journal of Veterinary Internal Medicine 36(2): 488-492.
- Rossi G et al (2020). Rapid resolution of large bowel diarrhea after the administration of a combination of a high-fiber diet and a probiotic mixture in 30 dogs, Veterinary Sciences 7(1): 21
- Lecoindre P and Gaschen FP (2011). Chronic idiopathic large bowel diarrhea in the dog, Veterinary Clinics of North America: Small Animal Practice 41(2): 447-456.
- Leib MS (2000). Treatment of chronic idiopathic large-bowel diarrhea in dogs with a highly digestible diet and soluble fiber: a retrospective review of 37 cases, Journal of Veterinary Internal Medicine 14(1): 27-32.
- Freeman L et al (2011). WSAVA nutritional assessment guidelines, Journal of Small Animal Practice 52(7): 385-396.
- Horvath-Ungerboeck C et al (2017). Detection of DNA from undeclared animal species in commercial elimination diets for dogs using PCR, Veterinary Dermatology 28(4): 373.
- Ricci R et al (2013). Identification of undeclared sources of animal origin in canine dry foods used in dietary elimination trials, Journal of Animal Physiology and Animal Nutrition 97(Suppl 1): 32-38.
- Raditic DM et al (2011). ELISA testing for common food antigens in four dry dog foods used in dietary elimination trials, Journal of Animal Physiology and Animal Nutrition 95(1): 90-97.
- Fossati LA et al (2019). Determination of mammalian DNA in commercial canine diets with uncommon and limited ingredients, Veterinary Medicine and Science 5(1): 30-38.
- Parr JM and Remillard RL (2014). Common confounders of dietary elimination trials contain the antigens soy, pork, and beef, Journal of the American Animal Hospital Association 50(5): 298-304.
- Toresson L et al (2016). Oral cobalamin supplementation in dogs with chronic enteropathies and hypocobalaminemia, Journal of Veterinary Internal Medicine 30(1): 101-107.
- Toresson L et al (2017). Oral cobalamin supplementation in cats with hypocobalaminaemia: a retrospective study, Journal of Feline Medicine and Surgery 19(12): 1,302-1,306.
- Stockman J et al (2013). Evaluation of recipes of home-prepared maintenance diets for dogs, Journal of the American Veterinary Medical Association 242(11): 1,500-1,505.
- Wilson SA et al (2019). Evaluation of the nutritional adequacy of recipes for home-prepared maintenance diets for cats, Journal of the American Veterinary Medical Association 254(10): 1,172-1,179.
- Laflamme D (1997). Development and validation of a body condition score system for dogs, Canine Practice 22(4): 10-15.
- Laflamme D (1997). Development and validation of a body condition score system for cats: a clinical tool, Feline Practice 25(5-6): 13-18.
- Salt C et al (2017). Growth standard charts for monitoring bodyweight in dogs of different sizes, Plos One 12(9): e0182064.
- Gross KL et al (2010). Macronutrients. In Hand MS et al (eds), Small Animal Clinical Nutrition (5th edn), Mark Morris Institute, Topeka: 49-105.
