30 Sept 2019
Genetics of disc disease in chondrodystrophic dogs
Nicolas Granger and Helene Vandenberghe discuss new insights into this condition and the genetics that cause it.
Intervertebral disc herniation is the most frequently encountered cause of spinal cord injury in dogs. It occurs largely because of chondroid metaplasia in genetically predisposed breeds, leading to disc herniation and various degrees of neurological deficits.
This article aims at briefly refreshing colleagues on the pathophysiology underlying disc extrusion, but mainly reviewing recent genetic advances and breed predisposition to intervertebral disc disease. This information is not readily available in the veterinary literature because it is published in more fundamental science journals.
Maintaining awareness of genetic discoveries is important because veterinarians and breeders may be able to use this knowledge to reduce the incidence of the disease in the near future.
Intervertebral disc disease classically affects chondrodystrophic dogs (Ghosh et al, 1977).
The chondroid metaplasia causes calcification and acute extrusion of the nucleus, leading to spinal cord dysfunction in dogs, such as paresis/paralysis and incontinence (Brisson, 2010).
Approximately 2% of dogs are affected, with approximately 15% showing the most severe signs, including loss of pain sensation (Granger and Carwardine, 2014).
A recent study by Brown et al (2017), from the University of California, Davis (UC Davis) School of Veterinary Medicine, suggested genetics could help to reduce the prevalence of the disease.
Here, the authors wish to briefly review intervertebral disc disease in dogs and update colleagues about these recent genetic advances.
Pathophysiology and clinical signs
The intervertebral discs that lie between adjacent vertebrae and cartilaginous end plates are responsible for the flexibility of the vertebral column. The intervertebral disc is composed of an outer fibrous ring, the annulus fibrosus and an inner gel-like centre, the nucleus pulposus.
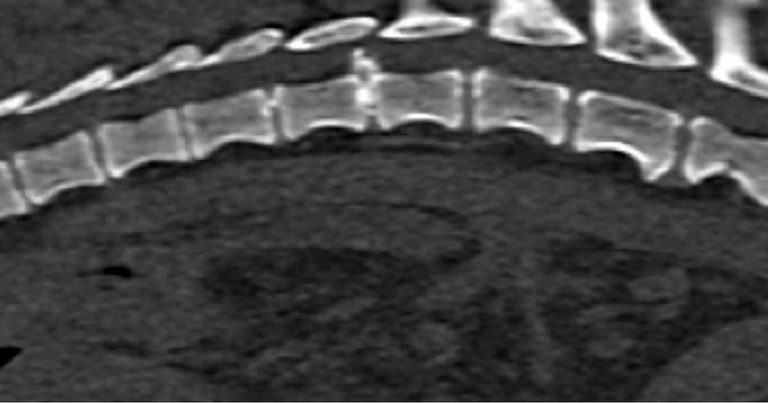
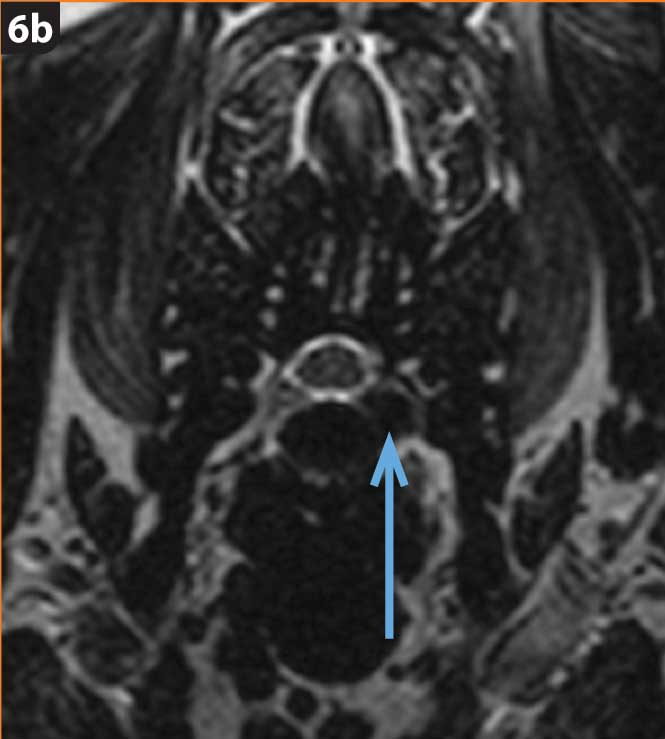
In chondrodystrophic dogs, but others as well, the nucleus pulposus undergoes a process of chondroid metaplasia, in which it is replaced by chondrocyte-like cells leading to calcification (Ghosh et al, 1977; Figures 1 to 3). This occurs typically before one year of age, whereas it is observed at an older age in non-chondrodystrophic breeds (Hansen et al, 2017).
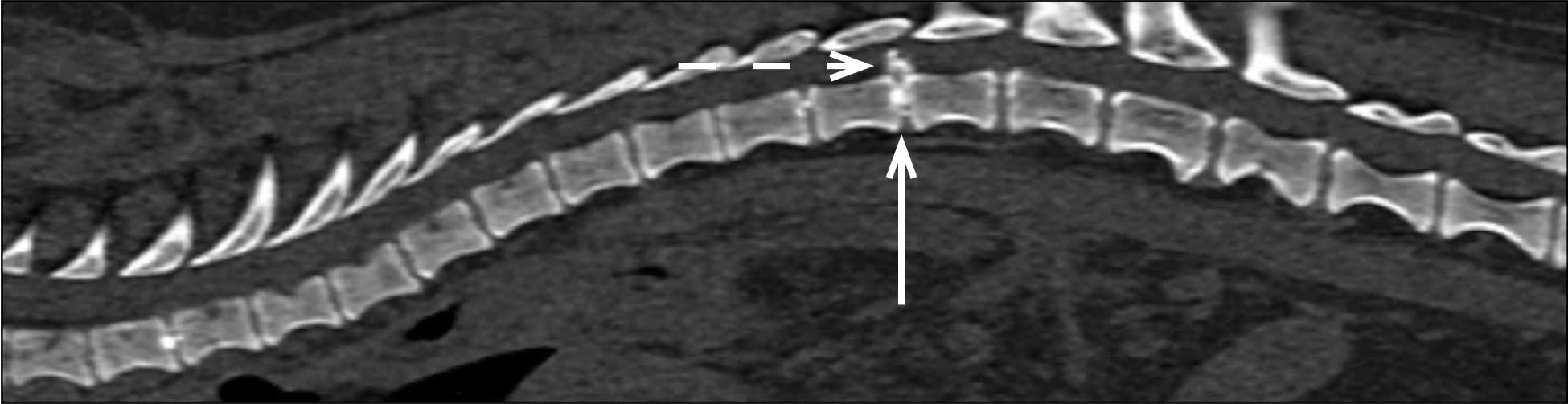
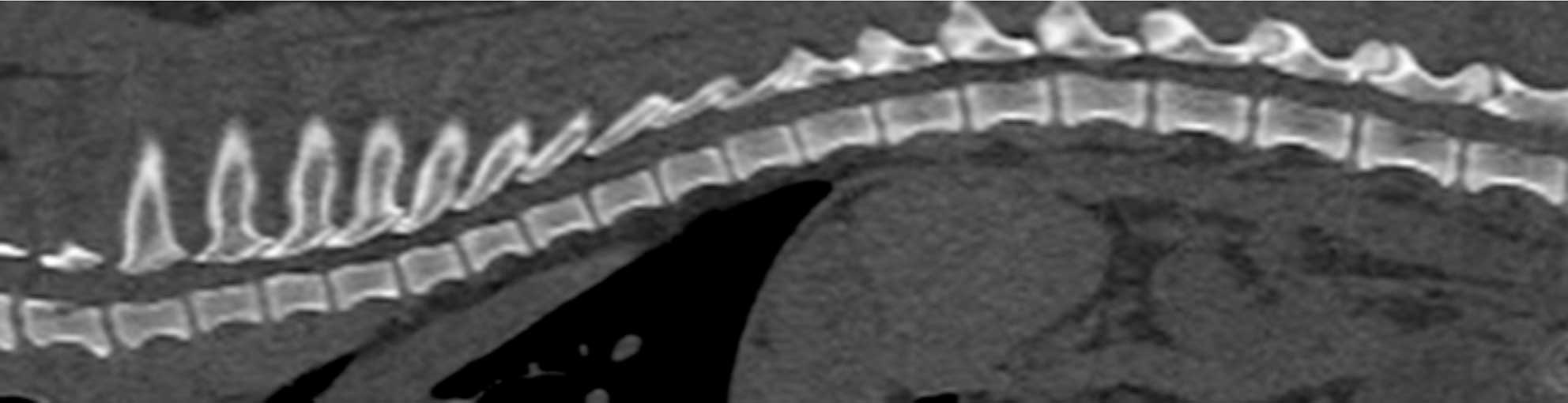
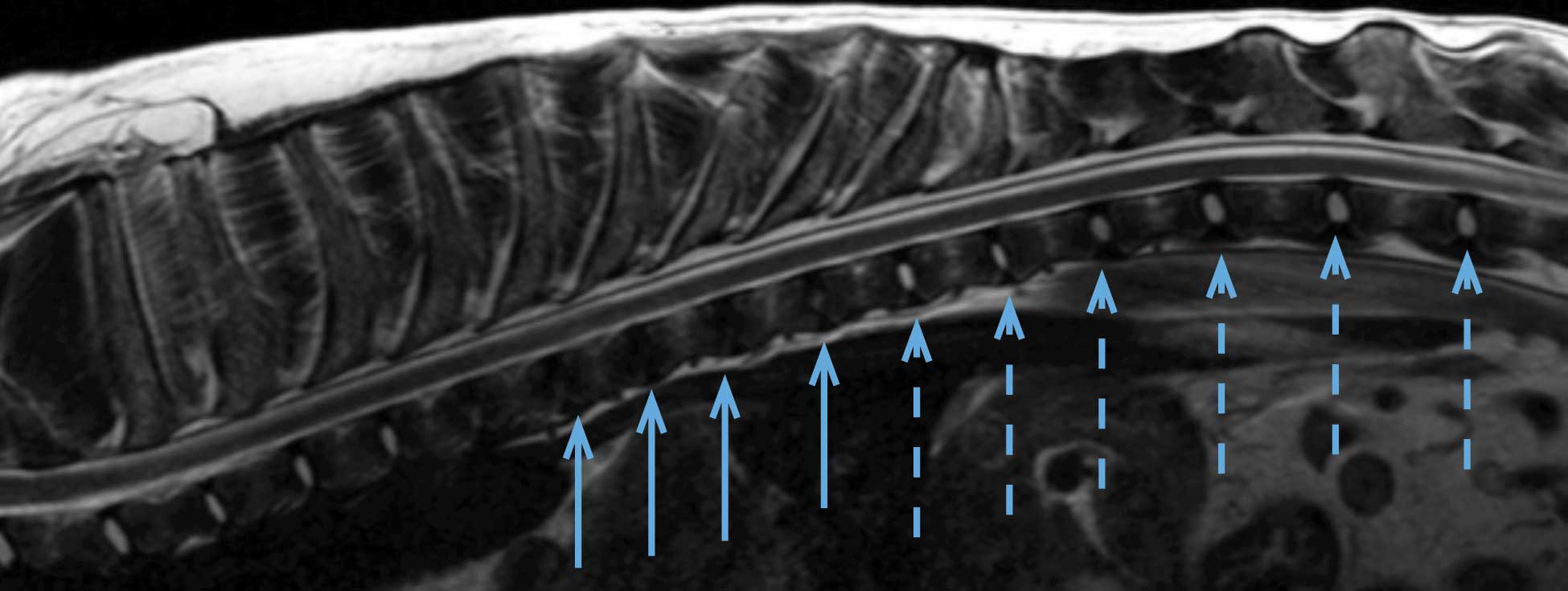
Hansen type-one intervertebral disc herniation (Hansen, 1952) is caused by an acute herniation of the degenerated calcified nucleus pulposus through the annulus fibrosus (Figures 4 and 5).
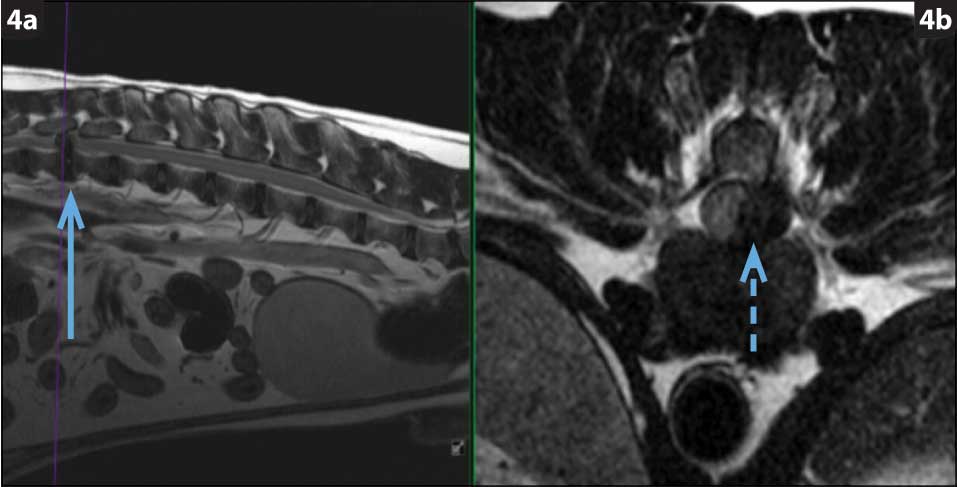
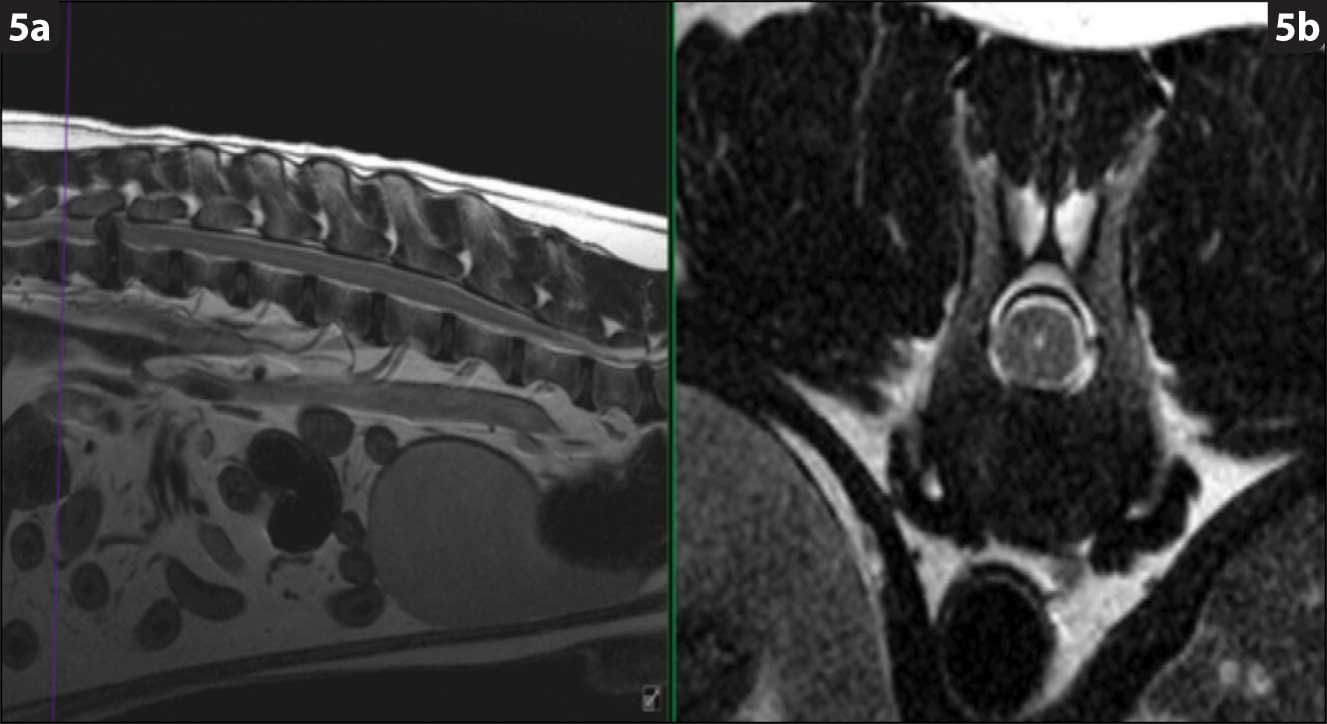
This has two consequences:
- severe spinal cord contusion caused – usually – by the high velocity of disc fragments migrating from the nucleus into the spinal canal
- a variable degree of spinal cord compression.
The clinical signs are likely to be caused by the contusive injury, in the initial stage (Jeffery and Freeman, 2018), because the ischaemic and biochemical events that occur directly affect cell membranes and disrupt the spinal cord parenchyma. The compression, although damaging axons, has less dramatic consequences in the acute phase.
Clinical signs range from severe spinal pain to neurological deficits, such as paresis/paralysis, urinary and faecal incontinence, and loss of sensation in the hindlimbs (for lesions of the thoracolumbar spinal cord segments; note dogs with cervical lesions will lose respiratory function before losing “deep pain” sensation and are, therefore, rarely seen with absent deep pain sensation in the forelimbs).
Surgical intervention is often chosen to relieve compression on the spinal cord in patients that lose the ability to walk. In a recent meta-analysis studying thoracolumbar disc extrusion and effect of spinal cord decompression, a clear trend existed to a greater proportion of dogs recovering and returning faster to ambulation for dogs treated with surgery, in comparison with dogs treated conservatively (Langerhuus and Miles, 2017); however, others have questioned this assumption with several valid lines of evidence (Jeffery and Freeman, 2018).
In particular, careful review of the literature shows of 951 dogs selected from 11 studies and undergoing disc fenestration “only” (that is, without direct spinal cord decompression) as a treatment for disc herniation, 94% of dogs with intact pain sensation recovered and 45% of dogs with no pain sensation recovered (Jeffery and Freeman, 2018). This is very similar to recovery rates put forward in studies looking at the effect of decompressive surgery for treatment of disc herniation (Langerhuus and Miles, 2017).
Identification of responsible mutation
The chondrodystrophic phenotype is defined by dysplastic and shortened long bones, together with early degeneration of intervertebral discs. Chondrodystrophic breeds include the dachshund (Figure 6), French bulldog, Pekingese, beagle, Lhasa apso, shih-tzu, Welsh corgi (Pembroke) and cocker spaniel (Bergknut et al, 2012).
In the dachshund, intervertebral disc disease represents a major health concern. The relative risk of the intervertebral disc degeneration-related diseases is 10 to 12 times higher than in other breeds. Around a fifth of dachshunds show clinical signs related to disc disease during their lives (Packer et al, 2016).
Long-term complications include incontinence and permanent neurological impairment. The overall mortality rate associated with the disease is 9.4 deaths/10,000 dog-years at risk (Bergknut et al, 2012). In about 25% of affected dachshunds, the disease leads to death or euthanasia (Bergknut et al, 2012).
Chondrodystrophic dogs also display skeletal dysplasia, presenting with very short legs. Defects of endochondral ossification in the long bones – and, to a lesser extent, in the vertebral bodies – have been demonstrated by histopathological analysis (Hansen, 1952).
Fribroblast growth factor (FGF) genes are key for appropriate growth and development, and FGF-associated mutations are implicated in human achondroplasia. People with achondroplasia are short in stature and prone to intervertebral disc herniation.
FGF4 is a growth factor gene. An FGF4 retrogene, originating from the parent FGF4 gene, has been found on chromosome 18 of the Canis familiaris genome (CFA18); this retrogene is abnormally inserted on CFA18 and seems to be responsible for small limb length in dogs, as shown by Parker et al (2009; Figure 7).
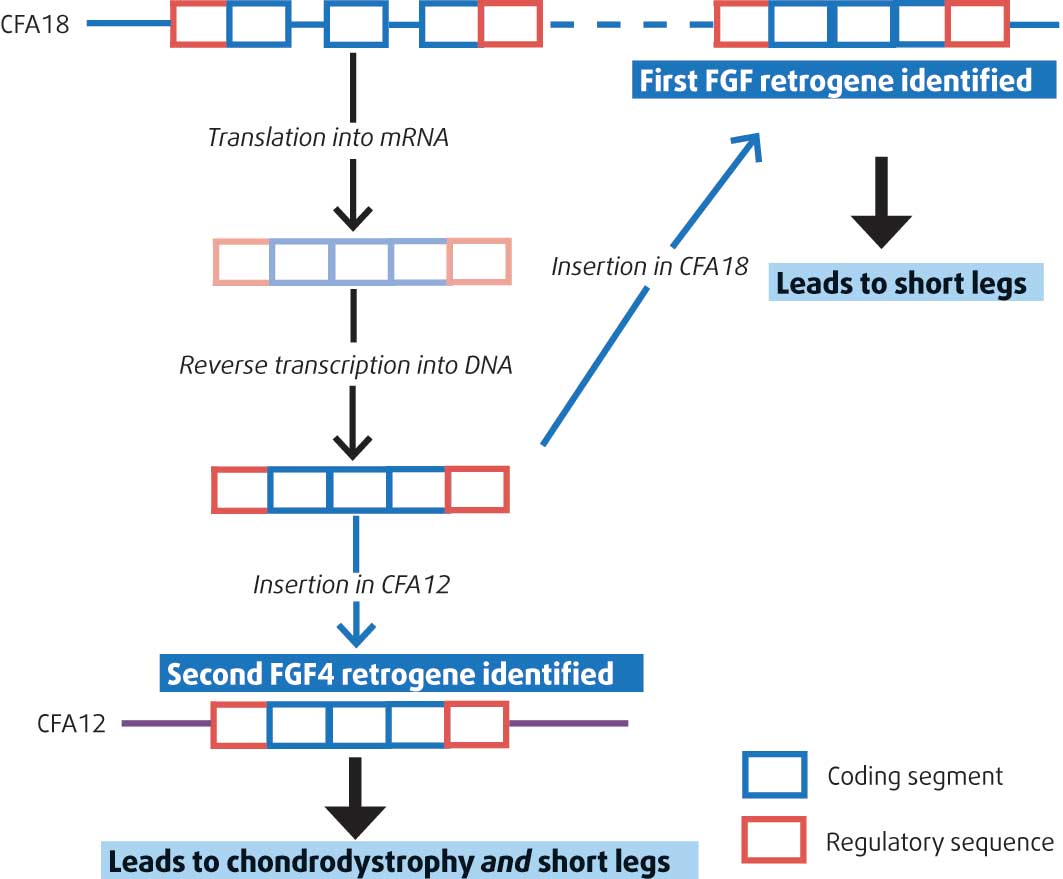
However, this insertion of FGF4 on CFA18 failed to explain the chondrodystrophoid phenotype in many breeds, including the French bulldog, American cocker spaniel and beagle, all known for their high susceptibility to intervertebral disc herniation.
Brown et al (2017) have now identified the presence of the FGF4 retrogene on another locus on Canis familiaris chromosome 12 (CFA12; Figure 7) and have associated this with both type-one intervertebral disc disease and chondrodystrophoid phenotype across many more dog breeds.
The authors (Brown et al, 2017) first identified that a region on CFA12 was associated with a segregating form of skeletal dysplasia in Nova Scotia duck tolling retrievers using a genome-wide association study.
The Nova Scotia duck tolling retriever is the smallest of the retriever dog breeds. As part of this study, this anomaly/haplotype was found to be shared by chondrodystrophic breeds.
Following that finding, a genome-wide association analysis searching for a cause for Hansen type-one intervertebral disc herniation identified the presence of the same abnormal region on CFA12 in chrondrodystrophic dogs. This suggested the locus is responsible for skeletal dysplasia in Nova Scotia duck tolling retrievers, and for the chondrodystrophoid phenotype and, consequently, Hansen type-one disc herniation for all dog breeds.
This insertion leads to a modification of the FGF4 expression in the neonatal intervertebral disc, also probably modifying the endochondral ossification in the embryo. In the mouse, FGF4 is expressed in various tissues during embryonic development. It is notably expressed in the somites and notochord that will form the vertebral column and the intervertebral discs. It explains, for the first time, both the short limb length and intervertebral disc disease, which is a significant finding.
Conclusion
Acute-onset myelopathy secondary to intervertebral disc herniation is the most common spinal cord disorder in dogs, with important health and financial consequences for owners and breeders. This can be partly explained by the increasing popularity of the chondrodystrophic breeds. The miniature dachshund is the most frequently affected breed.
The identification of a causative mutation (the insertion of the FGF4 retrogene) for intervertebral disc herniation – previously on CFA18 and now demonstrated to be present on CFA12 in association with the small stature of chondrodystrophic dogs – could be a useful information to reduce the disease incidence by breeding away from the FGF4 retrogene.
