29 Sept 2020
Heart disease in canine patients
Luca Ferasin describes diagnostic protocols for this issue, and treatment and management advice, including tips for dog owners.
- This article was originally published in Vet Times 50.40 (29 September 2020).
Canine cardiology has witnessed a remarkable evolution in the past decade.
Indeed, this period has been characterised by the publication of several large controlled studies that demonstrated the efficacy (or inefficacy) of different therapeutic interventions in the management of various cardiac diseases, both in symptomatic and asymptomatic patients.
Furthermore, guidelines for the management of canine cardiac disease have become available to assist clinicians in the diagnosis and treatment of these conditions.
This article briefly illustrates various diagnostic tests, treatment and management protocols in both symptomatic and asymptomatic dogs with heart disease, including advice for carers for efficient monitoring of their dogs at home.
Asymptomatic dogs
A dog with heart disease is classified as “asymptomatic” when the physical examination reveals the presence of an arrhythmia or an auscultatory abnormality (such as heart murmur, tachycardia, bradycardia, split heart sounds or gallop sounds) without clinical signs that could be associated with a cardiac disease (exercise intolerance, tachypnoea/dyspnoea, ascites, falling and fainting).
The recognition of an “asymptomatic” patient is of vital importance in modern cardiology and, in many cases, asymptomatic patients do not need immediate cardiac therapy, but only simple monitoring.
Asymptomatic patients with congenital defects
In human medicine, most congenital cardiac defects are diagnosed in utero during early pregnancy as part of antenatal screening programmes. Such early diagnosis prompts all the available interventions to correct the cardiac defects immediately or soon after birth.
However, in veterinary medicine, puppies and kittens are generally brought to the veterinarian for the first health check when they are approximately six to eight weeks of age, so only the congenital cardiac defects that are seen in small animal practice are those still asymptomatic and compatible with life, at least in the first months after birth. Indeed, many cases of stillborn or perinatal deaths may be associated with more severe cardiac defects that are not compatible with life.
If a heart murmur is detected during the first clinical assessment, this should prompt additional investigations – such as a Doppler echocardiographic exam – that, if performed by a qualified cardiologist or a very experienced clinician, reveals the nature and severity of the congenital defect.
Sometimes, less severe congenital defects are recognised later in life during pedigree breed screening schemes, performed with the intention of recognising potential carriers of the defect to avoid breeding from those subjects.
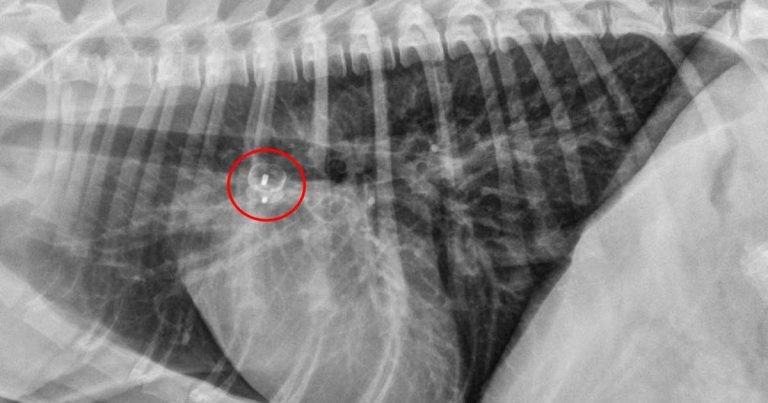
Cardiology specialists are trained to perform some interventions to correct life‑threatening congenital defects, either surgically or via catheterisation using minimally invasive procedures (Figures 1a and 1b).
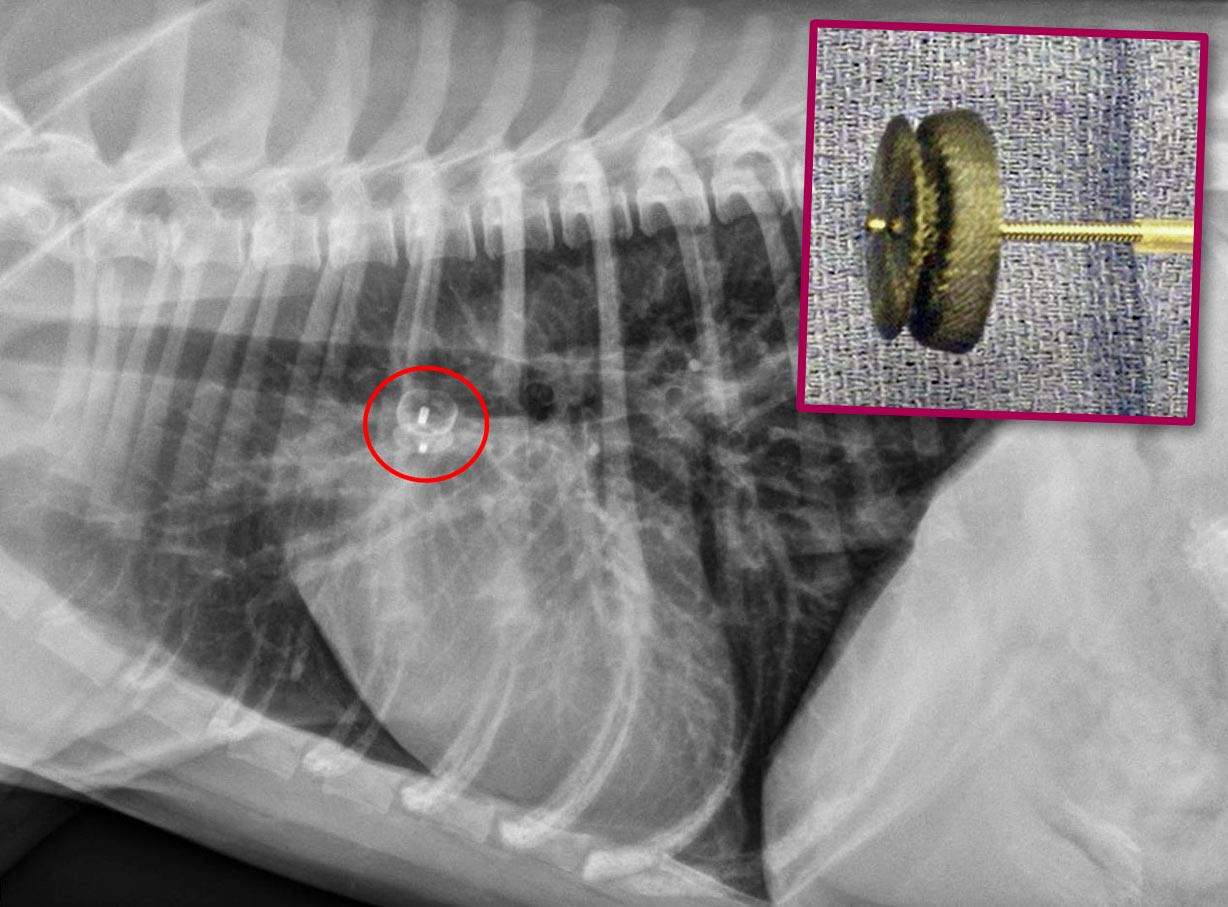
Patent ductus arteriosus
Patent ductus arteriosus (PDA) is probably one of the most common congenital cardiovascular abnormalities observed in dogs.
This defect usually leads to left-sided cardiac chamber dilation, arrhythmias, left-sided congestive heart failure (CHF) and, ultimately, death. However, it is also one of the few cardiac conditions that can be cured in small animal practice.
Thoracotomy and surgical ligation, in the hands of an experienced surgeon, is a well‑established and successful method for PDA closure in dogs. However, minimally invasive per-catheter procedures (canine duct occluder technique) have demonstrated a similar success rate and a more rapid recovery1.
Pulmonic stenosis
Pulmonic stenosis is another common congenital cardiac defect in dogs.
Doppler‑derived pressure gradients greater than 80mmHg across the pulmonic valve and/or significant right ventricular hypertrophy (RVH) classify the stenosis as “severe”, and affected dogs are at risk for developing clinical signs of exercise intolerance, syncope or sudden death.
Palliative balloon valvuloplasty is the best available therapy for this condition and has been recommended in severe cases with moderate or severe RVH, or when affected dogs are symptomatic2.
Aortic stenosis
Aortic stenosis in dogs is more commonly associated with a subvalvular fibrotic or fibromuscular ring in the left ventricular outflow tract (subaortic stenosis; SAS), and severe cases often develop exercise intolerance and malignant arrhythmias that may cause syncope and sudden death (typically within the first three or four years of life).
Several surgical procedures have been tried to remove the fibrous tissue in SAS cases, with disappointing results. However, the combination of balloon catheter dilation preceded by a balloon cutting technique to partially dissect the fibrous tissue is producing encouraging results3.
Diagnostic tests for asymptomatic patients
Auscultation
Cardiac auscultation represents one of the most powerful screening tests available in small animal cardiology. It is a simple, rapid and inexpensive technique available in every veterinary practice.
Auscultation can reveal the presence of a heart murmur or cardiac arrhythmia that can potentially be associated with an underlying cardiac disease.
Auscultation is invaluable for detecting congenital heart disease in puppies. The sensitivity of this test depends on clinical ability and experience, but the specificity is relatively low due to the fact many heart murmurs are not associated with a significant cardiac disease (flow or “innocent” murmurs).
The loudness of heart murmurs associated with congenital abnormalities tend to be proportionate to the severity of the underlying condition, as demonstrated in a recent study conducted in dogs4. A similar study confirmed the association between heart murmur intensity and degree of cardiac remodelling in mitral valve disease in dogs5.
Therefore, the presence of a moderate to loud murmur should always prompt additional investigations, such as echocardiographic examination.
Auscultation is also particularly useful in detecting arrhythmias often associated with myocardial disease in dogs.
Blood pressure measurement
Hypertension is a common, preventable risk factor for the development of cardiovascular disease and target organ damage (brain, myocardium, retina, endothelium, kidneys).
Screening protocols for hypertension should be considered in all geriatric dogs since they are more likely affected by systemic hypertension. Blood pressure measurement should be conducted following the American College of Veterinary Internal Medicine guidelines for the identification, evaluation and management of systemic hypertension.
In practice, errors may occur in measuring blood pressure as a result of instrument, observer or patient factors, but precision in identifying those with hypertension improves with the number of measurements taken6.
The relationship between blood pressure and cardiovascular risk is continuous, and this reiterates the importance of this invaluable screening test.
Echocardiography
Doppler echocardiographic examination has become widely available in veterinary practice to provide a non-invasive and reliable assessment of cardiac anatomy and function. For this reason, echocardiography represents one of the “gold standard” cardiac screening tests.
Learning how to perform a basic echocardiographic examination is very intuitive, and examiners can quickly recognise basic patterns of valvular or myocardial disease.
However, clinicians should be aware some echocardiographic changes may result from intraoperator and interoperator variability, which mainly depends on the operator skill and experience.
Furthermore, echocardiographic diagnosis of congenital cardiac defects is often very challenging as this also requires a deep knowledge of cardiac embryology, anatomy and pathophysiology; therefore, these challenging investigations should be reserved for qualified cardiologists.
Electrocardiography
Standard electrocardiography (ECG) and ambulatory 24‑hour ECG (Holter) recording represent other invaluable screening tests for occult myocardial disease both in humans and dogs.
When screening Dobermanns for occult dilated cardiomyopathy (DCM), the presence of at least 300 ventricular premature complexes (VPCs) in 24 hours is considered diagnostic, regardless of the concurrent echocardiographic findings. When Holter recording is not available, standard ECG showing at least 1 VPC in a five‑minute recording suggests 300 VPCs would be recorded in 24 hours if a Holter was performed.
Similarly, the presence of at least 100 VPCs in 24 hours in boxer dogs is highly suggestive of arrhythmogenic right ventricular cardiomyopathy (ARVC), especially if the VPCs are organised in complex arrhythmias.
Cardiac biomarkers
N-terminal prohormone of brain natriuretic peptide (NT-proBNP) has also been advocated as a screening test for DCM in Dobermanns where values greater than 500pmol/L can predict echocardiographic changes consistent with the occult disease.
In the same breed, values of cardiac troponin I (cTnI) greater than 0.22ng/ml (sensitivity 79.5%; specificity 84.4%) suggest the presence of occult DCM.
Antigen test for Dirofilaria immitis
Detection of circulating antigen of Dirofilaria immitis (heartworm disease) has represented the most useful screening test for heartworm disease in clinical practice for many years.
Several validated commercial antigen tests have demonstrated with a very good sensitivity and specificity (approaching 100%), and good positive predictive values, especially when used in patients for which heartworm infection is likely.
Genetic tests
Genetic tests are becoming very popular among pet carers and breeders, since they can be easily performed using a simple buccal swab.
Several genetic tests are available to screen for the following cardiac conditions:
- DCM in Dobermanns
- juvenile DCM in Portuguese water dogs
- ARVC in boxers
- subvalvular aortic stenosis7 in Newfoundlands
Screening panels also exist (for example, Canine HealthCheck) that screen dogs for multiple common inherited diseases affecting body metabolism and various organs.
Using a simple buccal swab, carers can receive a comprehensive report for more than 150 different genetic diseases and traits.
However, it should be emphasised genetic tests are not diagnostic tests and any abnormal result simply indicates the pet is a potential carrier or that an individual is at higher risk of developing a cardiac disease.
Management of asymptomatic patients
The principle of treating a symptomatic cardiac patient is rather straightforward. Once signs of congestion appear, clinical signs can be successfully controlled by cardiac medications and provide clinical improvement, sometimes for many months or even years.
Nevertheless, cardiac diseases are inevitably progressive, and patients will eventually die or will be euthanised due to signs of refractory CHF and unacceptable quality of life.
However, the principle of treating an asymptomatic patient is somewhat different since intervention is not aimed at controlling clinical signs, but prolonging life expectancy, improving the quality of life and, in some rare cases, correcting the underlying condition.
Until 2012, before the publication of the PROTECT Study to prove the efficacy of pimobendan in the treatment of asymptomatic DCM in Dobermanns, no evidence existed to support early medical intervention in asymptomatic patients with acquired cardiac disease before the onset of heart failure.
However, well-designed multicentre randomised controlled studies have been performed in the past decade to prove or disprove the efficacy of drugs commonly used in CHF, even in asymptomatic patients.
Pimobendan
In 2012, the results of a randomised, blinded, placebo-controlled, multicentre study involving 76 Dobermanns with preclinical DCM – recruited both in the UK and North America – showed the administration of pimobendan in this patient population with asymptomatic DCM can significantly extend the asymptomatic phase of the disease by nine months (primary end point; the PROTECT Study)8. Treatment with pimobendan also resulted in prolongation of the time to death attributable to all causes (secondary end point). This was the first study to prove the efficacy of a medical intervention in asymptomatic cardiac patients.
Following these exciting results, in 2016 the results of another trial (the EPIC Study) showed administration of pimobendan in dogs with myxomatous mitral valve disease (MMVD) and advanced signs of cardiac remodelling (both left ventricular and left atrial enlargement) can prolong the asymptomatic phase of these patients9.
Angiotensin-converting enzyme inhibitors
So far, two randomised, double-blinded, placebo-controlled studies that investigated the effect of early intervention in asymptomatic dogs with MMVD10,11 failed to demonstrate a significant increase in survival time or a delay in the onset of clinical signs.
Therefore, administration of angiotensin‑converting enzyme (ACE) inhibitors may not provide any beneficial effect in these patients, although many cardiologists still advocate the use of ACE inhibitors despite the lack of scientific evidence.
Spironolactone
For many years, the potential benefit of spironolactone to reduce cardiac remodelling has been advocated even in asymptomatic patients. However, this hypothesis has only been confirmed very recently, following the publication of the results of a large multicentre, randomised, placebo-controlled trial to evaluate the effect of combined spironolactone and an ACE inhibitor (benazepril) in asymptomatic dogs with MMVD.
This study demonstrated this combined medication can reduce cardiac remodelling based on vertebral heart score, left atrial size, ventricular diameter, transmitral inflow and NT-proBNP12.
Furosemide
No evidence exists to support early therapeutic intervention in dogs with asymptomatic MMVD.
Administration of furosemide in patients that are not in CHF should be avoided for the risk of dehydration, electrolyte imbalance (especially magnesium and potassium), renal hypoperfusion and further stimulation of the renin-angiotensin-aldosterone system (RAAS).
Beta blockers
So far, clinical trials addressing the efficacy of beta blockers for the treatment of asymptomatic dogs with mitral valve disease have failed to provide sufficient evidence to justify the use of these drugs.
Nevertheless, a minority of cardiologists recommend initiation of therapy with a low dosage of beta blockers, titrating to the highest tolerated dose over a period of approximately one to two months13.
Conservative management
Effective counselling and education of pet carers is important, and may enhance long‑term compliance with management strategies.
Simple explanations about the nature of the disease and signs of heart failure, including details on drug and other treatment strategies, are invaluable.
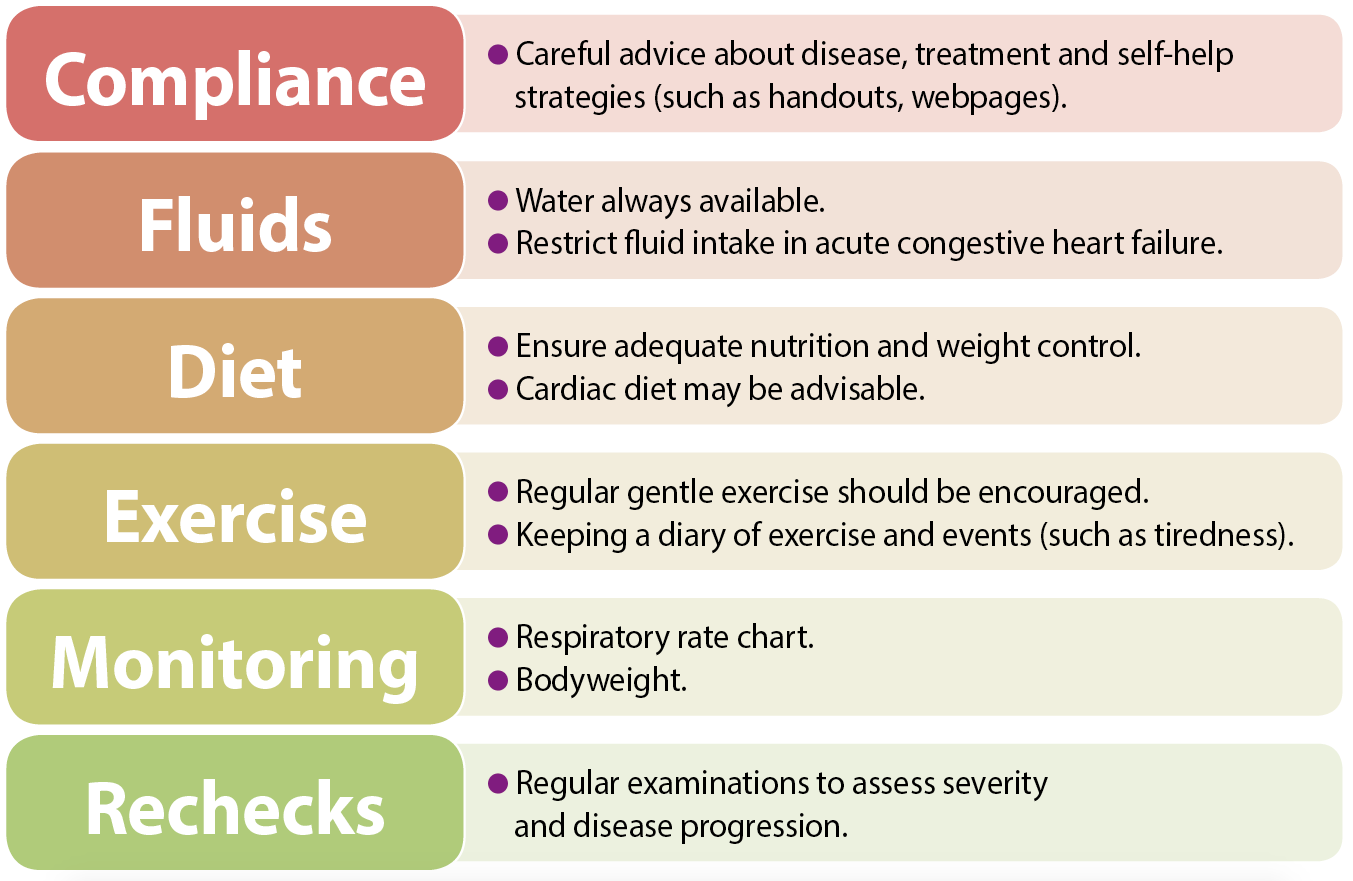
Emphasis should be placed on self-help strategies for each patient, and carers can be instructed how to monitor, for example, their pet’s weight, sleeping respiratory rate and exercise activity (Figure 2).
Symptomatic dogs
The most common clinical signs observed in dogs with heart failure (HF) – which is defined as a condition where the pumping ability of the heart (cardiac output) is no longer sufficient to satisfy the metabolic demand of the body tissues – include exercise intolerance, lethargy, inappetence, weight loss, respiratory changes and abdominal enlargement.
However, the most common clinical signs observed by carers of dogs with CHF are those associated with congestion, and are described as rapid and/or laboured breathing (tachypnoea and/or dyspnoea, respectively) or abdominal enlargement secondary to ascites.
Peripheral oedema observed in other species is not a common finding in dogs with CHF. Exercise intolerance and lethargy tend to appear before the onset of signs of congestion.
However, the transition from HF to CHF is not commonly observed by the pet’s carer, especially in sedentary dogs; therefore, the terms HF and CHF are often used interchangeably in small animal cardiology.
Tachypnoea/dyspnoea is primarily caused by the presence of pulmonary oedema and/or pleural effusion. Abdominal enlargement is the consequence of fluid accumulation in the peritoneal cavity (ascites).
In veterinary medicine, cough has been traditionally attributed to HF for decades. The cough reflex is an important physiological function to preserve the normal health of the respiratory tract and it is mostly initiated in the upper airways, which are extremely sensitive to mechanical stimulations, while lower airways are more chemosensitive.
In the lung periphery, at the bronchiolar and alveolar level, no coughing receptors have been reported. The importance of cough in maintaining respiratory health is evident in clinical situations where the cough reflex is ineffective, such as muscle weakness, laryngeal paralysis, tracheal collapse, bronchomalacia and chronic bronchitis.
Indeed, when any of these conditions are present, parenchymal atelectasis and/or recurrent pneumonia can occur.
Cough is not the result of pulmonary oedema, as commonly reported in many veterinary textbooks. This can be easily extrapolated by the fact the reflex is not present in the deeper respiratory tract (bronchioles and alveoli).
Instead, patients with pulmonary oedema will present with tachypnoea/dyspnoea. They may occasionally cough if a massive accumulation of fluid is present in the alveolar space, severe enough to reach higher airways and stimulate coughing receptors. However, this happens extremely rarely in our patients and it is usually limited to hyperacute cases, with a soft cough always accompanied by very severe dyspnoea.
For many years, cardiomegaly – and in particular left atrial enlargement – has been indicated as an important cause of coughing in dogs with mitral valve disease. However, in a study conducted in 206 dogs with mitral valve disease, a very weak association existed between cardiac size (measured on radiography and echocardiography) and the presence of cough14.
However, fluoroscopic studies conducted by the author in some (but not all) geriatric dogs with cardiomegaly have shown dynamic bronchial collapse synchronous with their heart beats and/or diaphragmatic movement.
Therefore, the most realistic explanation of this phenomenon is the concomitant presence of tracheobronchomalacia and cardiac enlargement. Indeed, young puppies with severe cardiomegaly due to congenital defects (for example, PDA) rarely cough, since their airways are healthy and do not collapse under the pressure of the enlarged heart.
In summary, dogs with cardiomegaly may cough, but the primary cause of this cough is likely to be an airway disease and this should be properly treated as airway disease, rather than heart failure.
A question often asked by practitioners is about the positive clinical response of coughing to cardiac medications. The most likely explanation for this correct observation is that dogs with cough caused by infectious agents tend to improve spontaneously.
Furthermore, furosemide has well-recognised antitussive properties, acting primarily at peripheral level, and without a placebo control group it is impossible to determine whether these dogs genuinely improve in response to cardiac medications.
Finally, it is realistic to believe that in dogs with severe cardiomegaly, furosemide can reduce the left atrial pressure and alleviate its compression on the adjacent airways.
Diagnostic tests for symptomatic patients
Echocardiography and point‑of‑care ultrasound
In analogy with the asymptomatic patient, Doppler echocardiographic examination represents the gold standard technique for the definitive diagnosis of the underlying cardiac disease and its staging.
However, recognition of signs of congestion (pulmonary oedema, pleural effusion, ascites) does not require a complete echocardiographic examination – and point-of care ultrasound (POCUS) examination techniques have recently been validated for the recognition of patterns of congestion without the need of thoracic radiography.
POCUS can be considered an extension of the physical examination, and in human medicine, lung ultrasound seems to exceed the sensitivity and specificity of chest auscultation and radiography for detection of pneumothorax, pleural effusion and lung consolidation.
Briefly, pulmonary oedema can be suspected when multiple simultaneous B-line artefacts (BLA) are observed during POCUS, while pleural effusion and ascites are confirmed by the presence of hypoechoic areas in the pleural space and abdominal cavity, respectively (Figure 3).
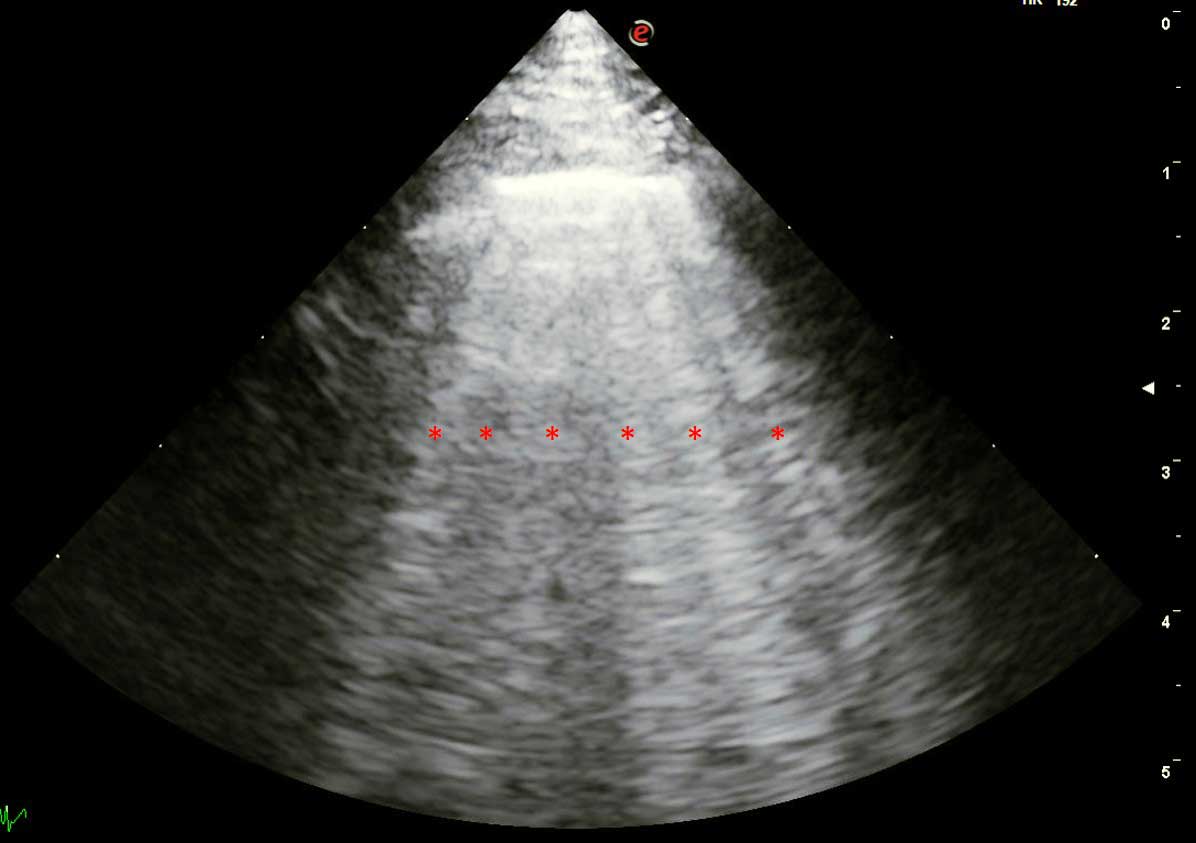
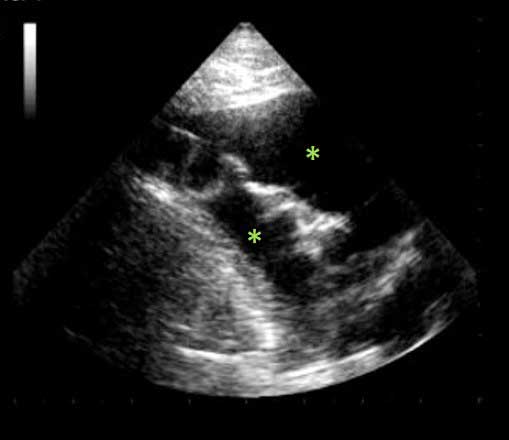
However, it should be emphasised that a correct interpretation of BLA in lung ultrasound is strongly influenced by associated ultrasonographic signs (such as left atrial enlargement) and careful integration of all relevant clinical information.
Thoracic radiography
Although thoracic radiography has become less fashionable following the introduction of POCUS, this diagnostic technique remains extremely important to support a POCUS diagnosis of pulmonary oedema and to identify non-cardiac causes of respiratory signs, such as tracheal and/or bronchial collapse, chronic bronchitis, lung neoplasia and pneumonia.
Despite the common tendency in practice to obtain thoracic radiographs in sedated or even anaesthetised patients, it should be noted most dogs would tolerate the procedure with a simple and gentle restraint with sandbags or radiotransparent positioners.
Cardiac biomarkers
In small animal cardiology, the two cardiac biomarkers most extensively used are cTnI and NT-proBNP.
In healthy dogs, little to no cTnI is detectable, but it is elevated in patients with significant heart disease associated with myocardial damage. Most veterinary tests have a relatively low limit of detection of approximately 0.2ng/ml, whereas circulating cTnI in dogs with mild cardiac disease (MMVD) are often less than 0.03ng/ml and can be detected only using newer high-sensitivity assays.
However, although elevated cardiac troponin is sensitive for the presence of myocardial injury, it is not specific to one underlying cause. Therefore, the use of this test to screen for specific HDs in various populations is limited, but it is an excellent test to quantify the extension of a myocardial damage.
NT-proBNP is a clinically useful marker of increased ventricular filling pressure, often observed in most cardiac disease.
Elevation of NT-proBNP is observed in most dogs with cardiac conditions, but it is not specific to any disease. Clinicians should pay careful attention to the interpretation of these results since normal NT‑proBNP values differ between canine breeds and can be elevated in renal disease, as well as hyperthyroidism, systemic and pulmonary hypertension, dynamic obstructions, and arrhythmias. Breed-specific reference intervals may improve the diagnostic accuracy of NT-proBNP measurement15.
ECG
ECG recording remains the only available diagnostic tool for the recognition and classification of cardiac arrhythmias and conduction disturbances. ECG is widely available in vet practices and its use is relatively simple, especially with more recent digital equipment.
Although ECG interpretation can often be challenging, various solutions are available to share exams and request specialist interpretation. Ambulatory (Holter) ECG recorders are also becoming widely available and they allow continuous monitoring of the electrical activity of the heart for 24 hours or more.
Holter monitors are also particularly useful to investigate episodes of falling/fainting, which can occasionally be seen in symptomatic dogs with advanced cardiac disease.
Management of symptomatic patients
Regardless of the underlying cardiac disease, dogs that present with signs of CHF should be treated with the following medications.
Loop diuretics
Loop diuretics (furosemide and torasemide) are effective in providing symptomatic relief and remain the first-line treatment, particularly in the presence of oedema.
They should be administered to effect (normalisation of the respiratory rate and/or radiographic resolution of pulmonary oedema).
Pimobendan
Pimobendan is a potent inotropic and vasodilatory agent (inodilator) that prolongs time to sudden death, euthanasia for cardiac reasons, or treatment failure in dogs with DCM or MMVD and CHF.
ACE inhibitors
ACE inhibitors have shown beneficial effects on mortality and quality of life in several prospective clinical trials, and are indicated in all stages of symptomatic CHF.
Spironolactone
Spironolactone is a specific pharmacological antagonist of aldosterone, which can be responsible for depression of baroreflex sensitivity, generalised vasoconstriction, myocardial hypertrophy and fibrosis, which – together with myocardial cell death – may underlie progressive adverse myocardial remodelling.
A published clinical trial has demonstrated spironolactone can decrease the risk of cardiac-related death, euthanasia and disease progression in dogs with moderate to severe MMVD12.
Cardiac surgery
Open heart cardiac valve repair in canine cardiology is still limited by elevated costs and relatively high surgical risk.
However, circumferential mitral annuloplasty and artificial chordal replacement appears to confer durability and improved long-term clinical outcome, with a survival of 93% three years post-surgery.
Clinical studies are also in progress to evaluate the efficacy of less invasive techniques.
Monitoring sleeping respiratory rate at home
In hospitalised dogs with CHF, resting respiratory rate has proven to be the most sensitive and specific single test for identifying left-sided CHF as a cause of respiratory signs and independently predicted CHF. Furthermore, resolution of the CHF resulted in respiratory rates returning to pre-CHF ranges.
However, sleeping respiratory rate (SRR) monitoring has shown to provide more consistent results – and most dogs and cats with stable, medically controlled CHF have SRR lower than 30 breaths per minute and relatively small day-to-day changes in SRR in the home environment.
These findings provide reasonable targets for clinicians to strive for when trying to manage cases of CHF, with the caveat that other factors (such as renal disease) should be considered when managing CHF.
Ongoing SRR measurements at home could also be used to adjust the diuretic to the lowest effective dose – in other words, the dose that maintains SRR lower than 30 breaths per minute, thereby reducing consequences of excessive diuresis16.
