11 Jan 2022
Heart disease in dogs
Ewelina Korzybska and Emily Dutton provide an overview of the most common conditions, including diagnostic and treatment modalities.
Approximately 10% of all canine patients in general practice have heart disease1, with acquired heart disease being much more common than congenital heart disease.
The two most common types of heart disease include myxomatous mitral valve disease (MMVD), affecting 49% of dogs; and dilated cardiomyopathy (DCM), affecting 21.1%2. The prevalence of congenital heart disease in dogs varies between 0.46% to 1.6%3,4.
MMVD
The most common acquired heart disease seen in practice is myxomatous valve disease, which can affect either the mitral valve alone, both the mitral and tricuspid valves, tricuspid valve, aortic valve, pulmonic valve, or a combination (Figure 1)5.
MMVD is detected in approximately 30% of dogs aged 13 years and older, and is responsible for about 75% of all cardiovascular disease in dogs1,6,7.
It is generally a slowly progressive disease of geriatric, small-breed dogs. Male dogs are affected more frequently than female dogs8. The disease is particularly common in the cavalier King Charles spaniel (CKCS), which is often affected relatively earlier in their life than dogs of other breeds9. Furthermore, 100% of CKCS older than 10 years of age will have MMVD10.
MMVD is characterised by nodular distortion of the valve leaflets, and sometimes thickening and lengthening of chordae tendineae. As the disease progresses, the valve leaflets become more nodular and result in mitral valve incompetence. Histologically, deposition of mucopolysaccharides within the valve leaflets with a fibrotic component may be present.
The mitral regurgitation may lead to an increase in the volume of blood in the left atrium, and over time to left atrial (LA) – and, therefore, left ventricular dilation.
Clinical presentation
On clinical presentation, a systolic left apical heart murmur may indicate the presence of disease. Often, the intensity of the murmur correlates with the severity of the disease. Dogs with soft heart murmurs (grade less than 3) are less likely to have cardiac enlargement and require treatment11,12. Dogs with heart murmurs grade 4 or more are more likely to have significant mitral valve insufficiency, and LA and ventricular dilation, and, therefore, require treatment11-13.
Imaging is, however, always recommended to stage the disease, according to the American College of Veterinary Internal Medicine (ACVIM) classification system (Table 1)14.
Common clinical signs associated with congestive heart failure (CHF) secondary to MMVD are an increased sleeping respiratory rate, breathlessness or exercise intolerance. Coughing is also reported as being present in some patients with heart failure; however, a cough has many respiratory causes, so the authors would always recommend diagnostic investigations to ascertain the cause of the cough.
The ACVIM consensus guidelines for the diagnosis and treatment of MMVD were updated in 2019 and advised dividing the disease into stages, as summarised in Table 1.
| Table 1. American College of Veterinary Internal Medicine classification of myxomatous mitral valve disease (MMVD) | |
|---|---|
| Grade | Description |
| A | Dogs at risk of developing MMVD. |
| B | Dogs with structural mitral valve disease, but have not yet developed signs of congestive heart failure (CHF). |
| B1 | Asymptomatic dogs with a heart murmur due to mitral regurgitation, but no radiographic or echocardiographic signs of cardiac remodelling. |
| B2 | Asymptomatic dogs with signs of cardiac remodelling secondary to MMVD. Echocardiographic values: left atrial:aortic ratio greater than or equal to 1.6 and normalized left ventricular internal dimension in diastole greater than or equal to 1.7. |
| C | Dogs with MMVD that have evidence of cardiac remodelling and either past or present CHF. |
| D | Dogs with MMVD, cardiac remodelling, CHF refractory to standard heart failure therapy. |
Diagnosis
Echocardiography is recommended to diagnose and monitor progression of MMVD. Basic parameters – including left ventricular systolic and diastolic diameter measured using M-mode, mitral regurgitant volume, and LA:aortic ratio – are crucial for assessment of the disease and monitoring of its progression.
Arrhythmias are identified in approximately 9% of dogs with MMVD. Atrial premature complexes, supraventricular tachycardia and atrial fibrillation are found to be most common in stage C MMVD, possibly due to atrial stretch12.
Older age, decreased appetite, lower body condition score, increased serum creatinine and alanine aminotransferase concentrations, and plasma natriuretic peptide concentration are all positively associated with the likelihood of being in stage B2 MMVD when compared to stage B113.
Accessible measurements, such as these, could be used to screen dogs with preclinical MMVD in general practice in future; however, validation studies would first be required. Encouraging at-risk dogs – such as those more likely to be stage B2 versus stage B1 to seek further echocardiographic evaluation – could result in a greater proportion of cases being appropriately managed.
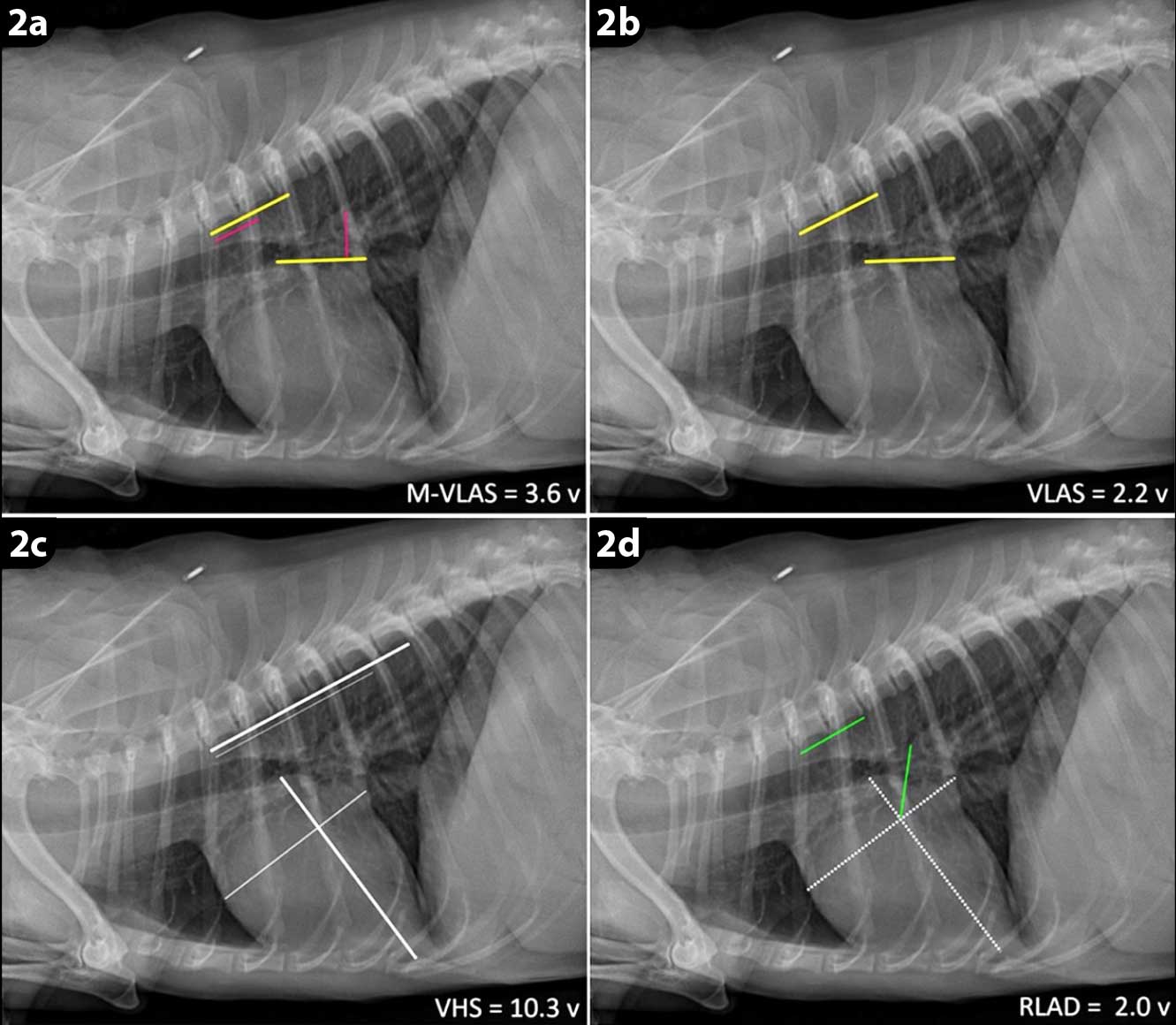
Diagnostic methods such as radiography could be used to support the diagnosis of cardiac enlargement and CHF. Vertebral heart score (VHS), vertebral LA size (VLAS), modified VLAS and radiographic LA dimension (RLAD) – described in Table 2 – may be used in a first opinion practice as a simple, cost-effective diagnostic method15,16 (Figure 2). RLAD was the most specific and sensitive test when compared to other radiographic methods of detecting cardiac enlargement15-17.
| Table 2. Radiographic measurements of heart size | |
|---|---|
| Measurement | Description |
| Modified vertebral left atrial size (M-VLAS; Figure 2a) | M-VLAS is measured from the centre of the most ventral aspect of the carina to the intersection between the most caudal aspect of the left atrium (LA) and the dorsal border of the caudal vena cava. A second measurement starts at the most distal LA border and perpendicularly meets the first line. These two separate lines are then drawn from the cranial edge of the T4 vertebrae extending in a caudal direction. The sum of the vertebral bodies (to the nearest 0.1 vertebrae) corresponding to the length of lines obtained from the left atrium consists of M-VLAS. |
| VLAS (Figure 2b) | A line is drawn from the centre of the ventral aspect of the carina to the caudal aspect of the left atrium, where it intersected with the dorsal border of the caudal vena cava. A line that is equal in length to the first was drawn at the cranial edge of T4 and extended caudally. |
| Vertebral heart sum (VHS; Figure 2c) | The long axis of the heart is measured from the ventral border of the left main stem bronchus to the cardiac apex. The maximal short axis of the heart is perpendicular to the long axis. The short and long axis dimensions are then added and compared to vertebral length, starting from the T4 vertebrae. |
| Radiographic left atrial dimension (RLAD; Figure 2d) | VHS is used as a baseline to measure RLAD. A line bisecting the 90° angle formed by the intersection of the VHS lines was drawn from this point to the radiographic projection of the dorsal edge of the LA. |
Cardiomegaly can be radiographically detected by measuring VHS. However, one study showed that cardiomegaly was identified by VHS in 33% of the dogs with normal echocardiographic LA and left ventricular chamber dimensions. In addition, the VHS failed to identify cardiomegaly in 14% to 21% of dogs with echocardiographic LA and left ventricular enlargement12.
Patients with MMVD often have concurrent bronchial or tracheal disease, so radiographs may be useful and as a baseline for future radiographs for when the dog becomes symptomatic.
Treatment
In early stages A and B1 of MMVD, medical treatment is not recommended. In stage B1, annual echocardiographic examinations may be indicated to monitor the progression of the disease.
Medical treatment should be initiated in stage B2 MMVD with pimobendan. The results of a prospective study showed that pimobendan delays the onset of CHF, cardiac-related death or euthanasia by approximately 15 months when compared to a control group1. The recommended dose in the treatment of MMVD varies between 0.25mg/kg to 0.3mg/kg orally every 12 hours, administered one hour before food.
The DELAY study demonstrated that combined administration of spironolactone and benazepril, with no other cardiac medications, does not delay the onset of heart failure in dogs with preclinical MMVD. Therefore, no other medical treatment is currently recommended during stage B2 MMVD18.
In stage C, the use of pimobendan with a loop diuretic is strongly recommended. Furosemide is the most often used loop diuretic, although torasemide is now being used more frequently since the results of two studies were published19,20 and since being proposed in the ACVIM consensus guidelines14.
Torasemide is an alternative loop diuretic to furosemide. One major benefit of torasemide over furosemide for the treatment of MMVD patients with heart failure is that it only needs to be administered once daily, which can be more practical for owners. Torasemide was also proven to prolong the time to – and decrease the risk of – cardiac-related death when compared to furosemide during long‑term administration19. The injectable form is not yet available in the UK.
The latest study comparing the treatment of CHF with either triple therapy (pimobendan, furosemide and an angiotensin-converting enzyme inhibitor [ACEi]) or dual therapy (pimobendan and furosemide alone) showed that addition of an ACEi (ramipril) did not have any beneficial effect on survival time21. Therefore, if financial constraints exist, pimobendan and a loop diuretic should be prioritised over triple therapy in the treatment of CHF.
Spironolactone can also be used in stage C MMVD, although a moderate level of evidence supports the treatment with spironolactone22,23. In the latest study, its addition to an ACEi gave better results in survival time in dogs with CHF than in dogs treated with an ACEi alone22. Unfortunately, in this study other medications commonly used in stage C – such as pimobendan – were restricted, while the ACEi used in this study in combination with spironolactone is not currently recommended in stage C MMVD21,22.
Atrial arrhythmias can be caused by LA stretch secondary to valvular incompetence. In cases complicated by atrial fibrillation, diltiazem (often in combination with digoxin) can be used to control ventricular rate24.
It is worth remembering that the option of mitral valve repair surgery exists, although it is not yet a widely used treatment option due to high costs and the limited number of referral hospitals where the procedure can be performed.
DCM
DCM is the second most common acquired heart condition seen in dogs. The disease is characterised by ventricular dilatation and impaired contractility, but an arrhythmic form is also common in boxers, called arrhythmogenic right ventricular cardiomyopathy.
The preclinical phase of DCM can last a long time (often years) with the clinical phase being very short. Treatment during the preclinical phase has been shown to prolong life in Dobermanns and Irish wolfhounds25,26, therefore highlighting the importance of early detection of this heart disease.
Idiopathic DCM is most commonly seen in large‑breed dogs such as Dobermanns, with a prevalence of 58%27. It is also very common in Irish wolfhounds, with a prevalence reported between 24% and 29%26; and deerhounds, with a prevalence of 21.6%28. In great Danes, prevalence varies between 11% and 35%, depending on the study29.
DCM is less commonly diagnosed in smaller breed dogs. In English cocker spaniels, DCM has been associated with low taurine levels, with a reported improvement in systolic function and reduced left ventricular dimensions following taurine supplementation and conventional cardiac therapy30.
A juvenile form of DCM has been reported in Portuguese water dogs, being a cause of mortality between 2 and 32 weeks of age.
DCM affects male dogs more often than female dogs, and is a disease of middle-aged and older dogs31.
Non-conventional diets, myocarditis and chronic tachycardias have been associated with dilated, poorly contractile hearts detected echocardiographically, which can mimic idiopathic DCM. However, these echocardiographic changes are often (but not always) reversible with an appropriate diet change, treatment of the underlying condition or heart rate control if caught and treated early enough.
In 2018, the US Food and Drug Administration (FDA) released a report of a potential connection between diet and DCM in dogs. The FDA reported on cases of DCM in dogs eating pet foods labelled as grain-free diets containing a high proportion of lentils, peas, other pulses or potatoes.
Recently, increased concern has been raised regarding the relationship of grain-free diets and those containing novel proteins to DCM phenotypes in dogs32,33. However, no definitive link has been established and a reported improvement of systolic function occurred after a diet change34.
Clinical presentation
The preclinical phase of idiopathic DCM can last years without any clinical signs.
Often, DCM is only diagnosed when dogs are experiencing heart failure and once they only have weeks to live. Soft systolic heart murmurs or arrhythmias can be detected on clinical examination, although these are not always present. Jugular pulses and a fluid thrill on abdominal ballottement can be present during clinical examination356, particularly if atrial fibrillation is present.
During the clinical phase, exercise intolerance, tachypnoea, syncope, heart murmurs, arrhythmias, decreased heart sound intensity, jugular distension, ascites, hypothermia or pulmonary crackles may be detected on clinical examination.
Diagnosis
Echocardiography is essential to assess systolic function of the heart, chamber size and dilatation.
Referring to breed‑specific reference intervals (RIs) is important when assessing heart size. Sighthounds, for example, have larger hearts than non-sighthound breeds, when scaled to bodyweight. It is, therefore, vital to refer to echocardiographic RIs when assessing the heart size in sighthound breeds to avoid wrongly diagnosing cardiomegaly28,36-38.
It is important to exclude other causes of a poorly contractile heart – for example, low circulating taurine concentrations, nutritional cardiomyopathy and hypothyroidism. However, a study on Dobermanns did not confirm the role of hypothyroidism in the aetiology or progression of DCM and the treatment of hypothyroidism did not improve the clinical outcome39.
Holter monitoring for 24 hours, to exclude a tachycardia‑induced cardiomyopathy, should be considered.
Electrocardiography (ECG) is useful to identify arrhythmias, particularly ventricular arrhythmias such as ventricular premature complexes and ventricular tachycardia. Lone atrial fibrillation can also occur and be a precursor to DCM in certain breeds, such as Irish wolfhounds. Atrial fibrillation has been positively associated with the presence of ascites in dogs with MMVD as well as DCM35.
Holter monitoring is particularly useful in arrhythmogenic forms of DCM to determine if antiarrhythmic medications are required and to help exclude tachycardia‑induced cardiomyopathy. Radiography may not be helpful if the patient has the arrhythmogenic form of the disease as cardiac enlargement is not always present. However, combined results of clinical examination, ECG and radiography (VHS, VLAS) were recently shown to be useful in one study for predicting clinically important DCM in large‑breed dogs40.
Genetic tests may become useful in future for helping diagnose DCM in dogs. However, the genetics of canine DCM is complicated and it is likely that DCM will be caused by more than one genetic defect in different breeds of dog (such as polygenic or oligogenic inheritance)41,42.
The authors are carrying out research (The GENDEER Study) assessing whether a genetic basis exists to DCM in deerhounds – to learn more, visit www.deerhoundgenetics.com
Treatment
Treatment of DCM should include pimobendan; it is licensed for Dobermanns with preclinical DCM, dose range 0.25mg/kg to 0.3mg/kg orally twice daily. It was shown to delay the onset of heart failure or sudden cardiac death from 441 to 718 days25,26.
Benazepril was shown to prolong the disease free interval from 339 to 425 days27. Furosemide is commonly administered in patients that develop heart failure, at a dose of 2mg/kg orally twice daily.
With the arrhythmogenic form of cardiomyopathy, antiarrhythmic medications may need to be considered depending on the presence and complexity of ventricular arrhythmias. Medications may include sotalol (a class II/III antiarrhythmic drug) or mexiletine (a class Ib antiarrhythmic drug).
It is important to remember that every antiarrhythmic drug is also proarrhythmic, so they should be used with caution.
Unfortunately, no antiarrhythmic medications are licensed for dogs in the UK.
Congenital heart disease
Congenital heart disease is a significant cause of cardiovascular morbidity and mortality in small animals. In one retrospective study, 0.13% of a mixed‑breed population of 76,301 dogs were found to be affected by congenital heart disease44.
The most common congenital heart condition found in dogs is pulmonic stenosis (PS), followed by patent ductus arteriosus (PDA), aortic stenosis and ventricular septal defects.
PS
PS is by far the most common congenital heart disease in dogs, with approximately 31% of mixed‑breed dogs with congenital heart disease having PS44.
A prevalence of between 11 per cent to 21%, and 32% has been reported in different articles45,46. Popular breeds predisposed to PS include bulldogs, French bulldogs, miniature schnauzers, English cocker spaniels and boxers. PS is likely to be genetic in origin46,47.
The stenotic area most often affects the pulmonic valve and less often can be localised underneath the valve as a subvalvular stenosis or above the valve as a supravalvular PS45.
Two main types of valvular PS exist (Table 3), although the two forms are not mutually exclusive.
| Table 3. Types of pulmonic stenosis | |
|---|---|
| Type | Description |
| Type A | Fusion of the peripheral edges of the semilunar valves (commissural fusion) and post-stenotic dilatation of the pulmonary trunk. Type A is more prevalent. |
| Type B | May involve hypoplasia of the pulmonary annulus and pulmonary trunk, with the cusps appearing thickened and immobile. |
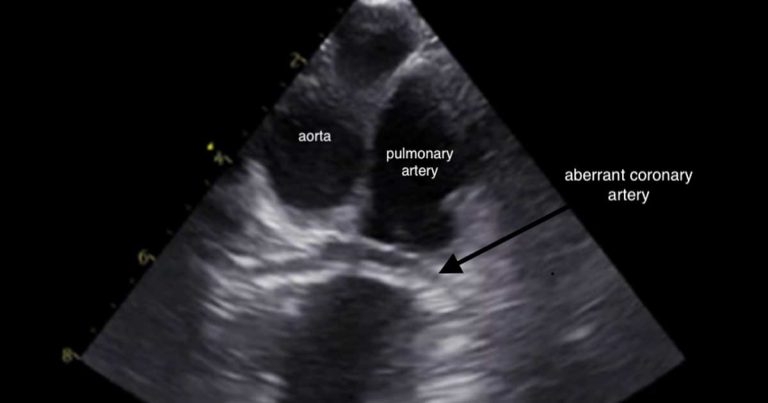
Brachycephalic breeds, particularly bulldogs, can have constriction of the pulmonary valve annulus or the subvalvular area by an aberrant coronary artery, such as with type R2A anomaly, creating a stenosis. It is important those patients with aberrant coronary arteries are identified as they are at an increased risk of rupture of the coronary artery and, therefore, fatal haemorrhage during balloon valvuloplasty.
Clinical presentation
Young dogs are often asymptomatic, but can experience exercise intolerance, syncope or right‑sided CHF. Severe concentric right ventricular hypertrophy caused by pressure overload may lead to myocardial hypoxia and ventricular arrhythmias. These patients may, therefore, die suddenly.
On clinical examination, a left‑sided, basilar, systolic heart murmur is a typical finding and may be the only sign of underlying congenital heart disease.
The severity of PS usually correlates with the grade of the murmur, with the higher murmur grades being associated with increased PS severity. Softer murmurs, on the other hand, in dogs with PS are indicative of mild lesions48.
Diagnosis
Echocardiography is essential to assess the right side of the heart.
Increased afterload causes concentric hypertrophy of the right ventricle. Examination of the pulmonic valve itself to assess any dysplastic or tethered leaflets is vital.
Post‑stenotic dilatation above the valve is also a common finding.
Spectral Doppler is used to assess the severity of PS, according to the speed of blood flow across the valve. This non‑invasive diagnostic procedure can determine the severity of the stenosis by estimating a pressure gradient across the obstruction. It can be classified as a mild (lower than 50mmHg), moderate (50mmHg to 80mmHg) or severe (greater than 80mmHg)49,50.
Transoesophageal echocardiography (Figure 3), as well as CT angiography, are used in some centres to aid diagnosis.
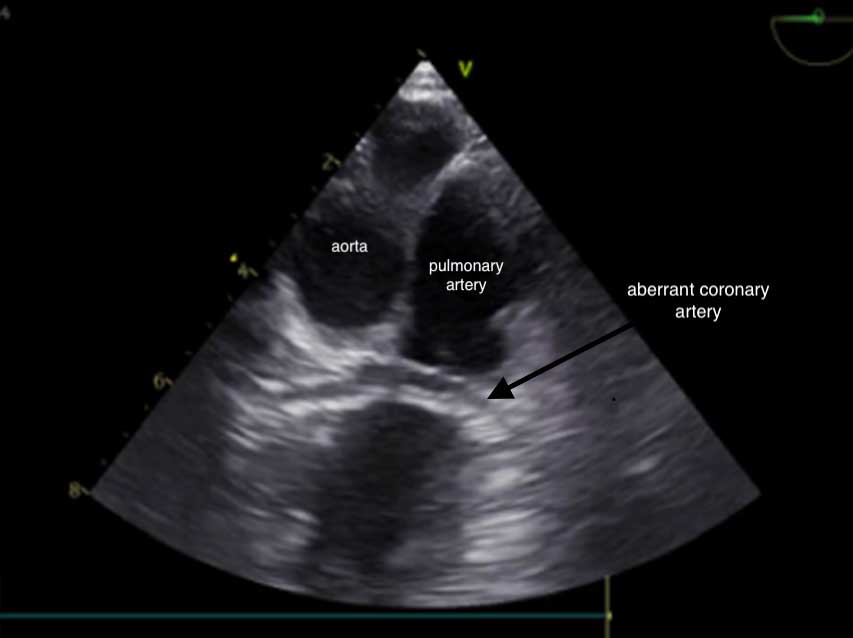
Recently published case reports have described placement of stents in dogs with severe PS and aberrant coronary arteries51. However, at the time of publication, no survival studies have been published showing a mortality benefit with stent placement.
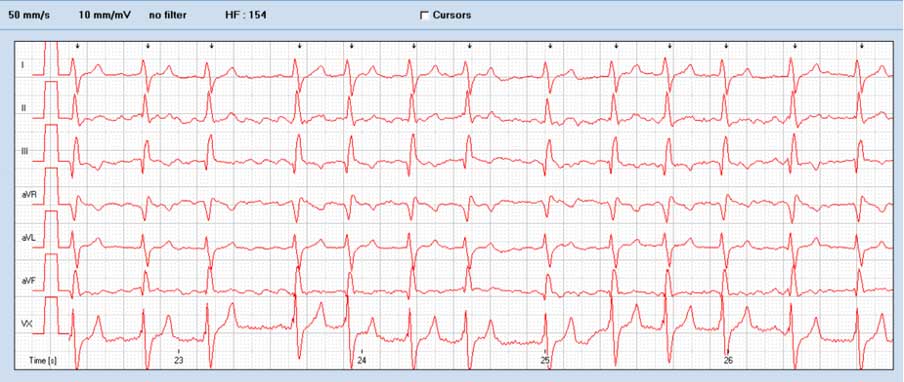
Electrocardiography often demonstrates right axis deviation characterised by small R waves and deep S waves. Atrial fibrillation is another possible finding in dogs with PS (Figures 4 and 5).
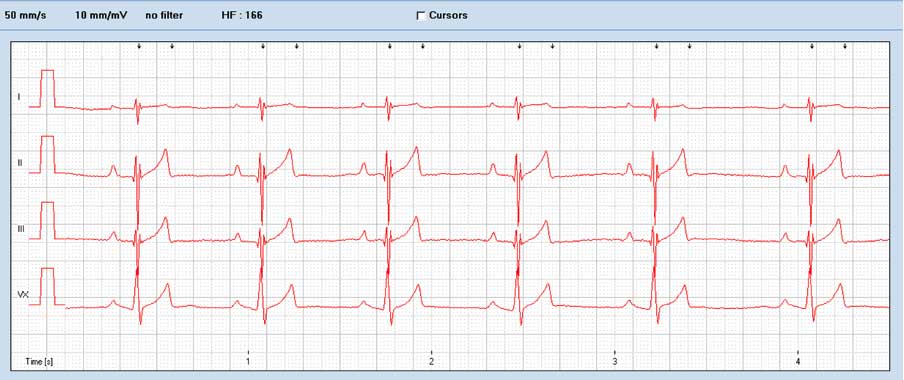
Treatment
Diagnosis and treatment of congenital heart disease should be initiated ideally before onset of heart failure as it alters the prognosis.
Beta-blockers are administered to those patients with moderate or severe PS (and no heart failure), to reduce myocardial oxygen demand and to improve coronary perfusion. Beta‑blockers may also help reduce the chance of sudden death through their antiarrhythmic effect. Atenolol is commonly prescribed at a dose of 1mg/kg orally every 24 hours45.
Medical therapy is usually started prior to surgical balloon valvuloplasty. During the procedure, an angiographic catheter is guided from a jugular or femoral vein approach and through the stenotic pulmonic valve. An inflated balloon is then used to break open the stenosis (Figure 6). The balloon valvuloplasty results in a significant decrease in pressure gradient.
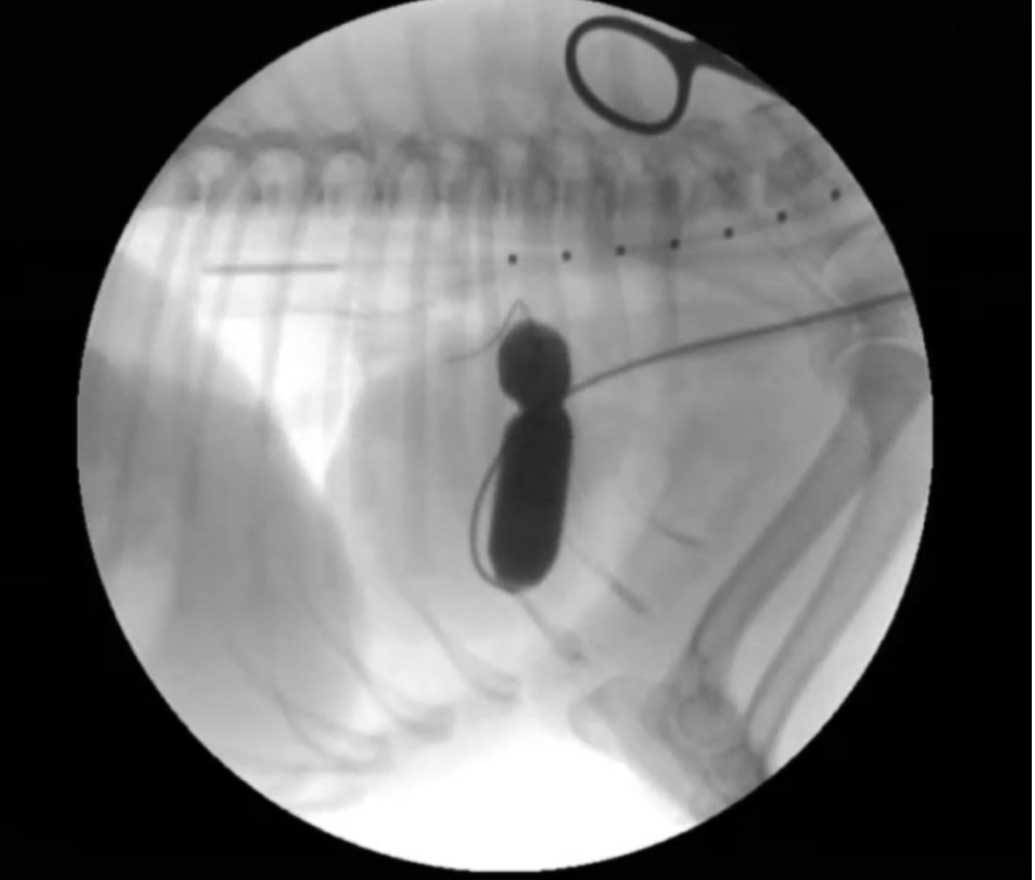
This procedure is recommended for patients with severe PS and has been shown to give a better outcome than medical treatment alone. The evidence is less clear for patients with moderate PS that have undergone a balloon valvuloplasty45.
PDA
PDA is the second most common congenital heart disease seen in dogs.
About 17% of congenital heart diseases in dogs in a mixed-breed population of 76,301 dogs included in the retrospective study had a PDA44. Another study revealed a prevalence of 20.9% in a population of 4,480 dogs46. Corgis, English cocker spaniels, border collies and German shepherd dogs are predisposed to PDA.
In the fetal circulation, the ductus arteriosus serves to bypass the non-functional lungs. PDA is a vessel connecting the aorta and pulmonary artery in the fetus that normally closes shortly after birth.
Female dogs are predisposed to having PDA. The defect has been shown to be transmitted as a polygenic trait52.
Clinical presentation
Dogs may be asymptomatic or present with severe CHF with left to right shunting PDAs.
A characteristic, continuous, “machinery” heart murmur can be detected on auscultation of left to right shunting PDAs. Volume overload can lead to left ventricular and atrial dilation, and may eventually lead to left-sided heart failure. The blood usually flows from left to right due to the pressure difference between the aorta and pulmonary artery.
Reversal of left to right shunts occurs rarely and usually early in life. It happens when pulmonary vascular resistance increases and exceeds systemic vascular resistance.
In large‑sized PDAs, excessive pulmonary blood flow can dramatically increase pulmonary vascular resistance, which leads to pulmonary hypertension, and shunt reversal may occur. Systemic hypoxaemia of the caudal half of the body can cause polycythaemia, hindlimb collapse or weakness during exertion, caudal cyanosis or organ failure.
Symptoms associated with the rare reverse shunting PDAs include disappearance of the heart murmur, arrhythmias, caudal cyanosis, collapse of the hindlimbs during exercise, or lethargy.
Diagnosis of left to right shunting PDA
Echocardiography findings of left to right shunting PDAs include eccentric hypertrophy of the left ventricle and LA enlargement due to volume overload.
Measurement of the ductus using colour Doppler and two‑dimensional echocardiography can be achieved from left and right parasternal views.
ECG can demonstrate tall R waves in lead II, indicative of enlargement of the left ventricle. A spectrum of cardiac arrhythmias may be seen, including both atrial and ventricular premature complexes, and atrial fibrillation.
Radiographic abnormalities may demonstrate LA and ventricular enlargement, prominent pulmonary vessels, a pulmonic bulge, aortic bulge and left auricular bulge, as well as the presence of pulmonary oedema.
Treatment
Angiography and transoesophageal echocardiography performed on anaesthetised patients is often used to determine the exact size of the occluding device that should be used.
Treatment options include the use of occluding devices or surgical ligation, depending on the shape of the PDA. “Funnel shaped” PDAs can be closed using occluding devices with a good success rate.
Occluding devices, such as the Amplatz canine duct occluder (ACDO) can be used in dogs from approximately 3kg when placed via the femoral artery (Figure 7). This method has been proven to be superior to other methods as the occlusion seems more complete, is associated with less complications and the procedure lasts shorter periods of time compared to other devices53. The remaining limiting factor with this device is the size of the patient53.
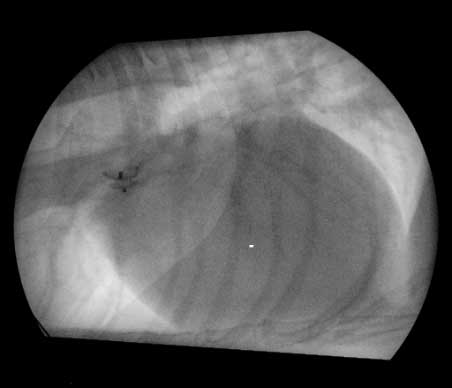
In smaller patients, accessing the heart via the transvenous route (the femoral vein) and using an Amplatzer vascular plug device for closure is often advised (Figure 8). Advantages of this device over the ACDO is that it can be delivered via a smaller catheter and it may be implanted via the femoral vein. This procedure consists of releasing the first disc at the level of the aorta and the middle disc in the PDA ampulla. The distal part is extruded at the pulmonary ostium level. Contrast angiography is then used to assess whether any flow is present across the PDA54,55.
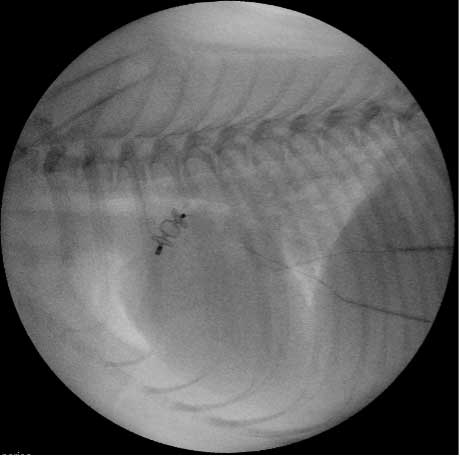
Minimally invasive (keyhole) techniques have more benefits when compared to surgical ligation (via thoracotomy), such as a reduced risk of haemorrhage or infection, reduced postoperative pain and the postoperative hospitalisation time often being reduced.
The prognosis after successfully closing a PDA is often excellent. Without the interventional surgery, the patient may develop heart failure during the first 12 months of life. After the procedure, the heart remodels and reduces in size. Although systolic dysfunction may remain, it does not usually affect life span56.
Conclusion
This article has reviewed the latest evidence‑based medicine for treatment of the most common heart diseases in canine patients.
The management of acute heart failure in dogs is not described in this article. For further information regarding this, refer to the ACVIM consensus guidelines14.
- Some drugs mentioned in this article are used under the cascade.
References
- Boswood A et al (2016). Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study – a randomized clinical trial, Journal of Veterinary Internal Medicine 30(6): 1,765-1,779.
- Baumgartner C and Glaus TM (2004). Acquired cardiac diseases in the dog: a retrospective analysis, Schweizer Archiv fur Tierheilkunde 146(9): 423-330.
- Buchanan JW (1990). Pulmonic stenosis caused by single coronary artery in dogs: four cases (1965-1984), Journal of the American Veterinary Medical Association 196(1): 115-120.
- Lucina SB et al (2021). Congenital heart disease in dogs: a retrospective study of 95 cases, Topics in Companion Animal Medicine 43: 100505.
- Zachary JF (2016). Pathologic Basis of Veterinary Disease – Expert Consult (6th edn), Mosby, Maryland Heights: 610.
- Kvart C and Häggström J (2000). Acquired valvular heart disease. In Ettinger SJ and Feldman CE (eds), Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat (5th edn), Saunders, Philadelphia: 787-800.
- Kahn CM (2005). The Merck Veterinary Manual (9th edn), Merck, Whitehouse Station: 90.
- Mattin M J et al (2015). Degenerative mitral valve disease: survival of dogs attending primary-care practice in England, Preventive Veterinary Medicine 122(4): 436-442.
- Reimann MJ et al (2017). Mitral regurgitation severity and left ventricular systolic dimension predict survival in young cavalier King Charles spaniels, Journal of Veterinary Internal Medicine 31(4): 1,008-1,016.
- Ramos JP et al (2021). Clinical and echocardiographic findings in an aged population of cavalier King Charles spaniels, Animals 11(4): 949.
- Ljungvall I et al (2014). Murmur intensity in small‑breed dogs with myxomatous mitral valve disease reflects disease severity, Journal of Small Animal Practice 55(11): 545-550.
- Franchini A et al (2021). The longitudinal outcome of canine (K9) myxomatous mitral valve disease (LOOK‑Mitral registry): baseline characteristics, Journal of Veterinary Cardiology 36: 32-47.
- Wilshaw J et al (2021). Accuracy of history, physical examination, cardiac biomarkers, and biochemical variables in identifying dogs with stage B2 degenerative mitral valve disease, Journal of Veterinary Internal Medicine 35(2): 755-770.
- Keene BW et al (2019). ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs, Journal of Veterinary Internal Medicine 33(3): 1,127-1,140.
- Malcolm EL et al (2018). Diagnostic value of vertebral left atrial size as determined from thoracic radiographs for assessment of left atrial size in dogs with myxomatous mitral valve disease, Journal of the American Veterinary Medical Association 253(8): 1,038-1,045.
- Lam C et al (2021). Radiographic quantification of left atrial size in dogs with myxomatous mitral valve disease, Journal of Veterinary Internal Medicine 35(2): 747-754.
- Salguero XS et al (2018). A radiographic measurement of left atrial size in dogs, Irish Veterinary Journal 71: 25.
- Borgarelli M et al (2020). DELay of Appearance of sYmptoms of canine degenerative mitral valve disease treated with spironolactone and benazepril: the DELAY Study, Journal of Veterinary Cardiology 27: 34-53.
- Besche B et al (2020). Efficacy of oral torasemide in dogs with degenerative mitral valve disease and new onset congestive heart failure: the CARPODIEM study, Journal of Veterinary Internal Medicine 34(5): 1,746-1,758.
- Chetboul V et al (2017). Short-term efficacy and safety of torasemide and furosemide in 366 dogs with degenerative mitral valve disease: the TEST study, Journal of Veterinary Internal Medicine 31(6): 1,629-1,642.
- Wess G et al (2020). Efficacy of adding ramipril (VAsotop) to the combination of furosemide (Lasix) and pimobendan (VEtmedin) in dogs with mitral valve degeneration: the VALVE trial, Journal of Veterinary Internal Medicine 34(6): 2,232-2,241.
- Coffman M et al (2021). Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: The BEnazepril Spironolactone STudy (BESST), Journal of Veterinary Internal Medicine 35(4): 1,673-1,687.
- Bernay F et al (2010). Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease, Journal of Veterinary Internal Medicine 24(2): 331-341.
- Gelzer ARM et al (2009). Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: a randomized crossover study in 18 dogs, Journal of Veterinary Internal Medicine 23(3): 499-508.
- Summerfield NJ et al (2012). Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman pinschers with preclinical dilated cardiomyopathy (the PROTECT Study), Journal of Veterinary Internal Medicine 26(6): 1,337-1,349.
- Vollmar AC and Fox PR (2016). Long-term outcome of Irish wolfhound dogs with preclinical cardiomyopathy, atrial fibrillation, or both treated with pimobendan, benazepril hydrochloride, or methyldigoxin monotherapy, Journal of Veterinary Internal Medicine 30(2): 553-559.
- Wess G et al (2010). Prevalence of dilated cardiomyopathy in Doberman pinschers in various age groups, Journal of Veterinary Internal Medicine 24(3): 533-538.
- Dutton E et al (2021). Echocardiographic reference intervals in healthy UK deerhounds and prevalence of preclinical dilated cardiomyopathy: a prospective, longitudinal study, Journal of Veterinary Cardiology [Epub ahead of publication], DOI: 10.1016/j.jvc.2021.04.001.
- Stephenson HM et al (2012). Screening for dilated cardiomyopathy in great Danes in the United Kingdom, Journal of Veterinary Internal Medicine 26(5): 1,140-1,147.
- Basili M et al (2021) Low plasma taurine levels in English cocker spaniels diagnosed with dilated cardiomyopathy, Journal of Small Animal Practice 62(7): 570-579.
- Oyama M (2015). Cardiomyopathy. In Smith F, Tilley L, Oyama M and Sleeper M (eds). Manual of Canine and Feline Cardiology (5th edn), Elsevier, St Louis: 141-151.
- McCauley SR et al (2020). Review of canine dilated cardiomyopathy in the wake of diet-associated concerns, Journal of Small Animal Sciences 98(6): 155.
- Freeman LM et al (2018). Diet-associated dilated cardiomyopathy in dogs: what do we know?, Journal of the American Veterinary Medical Association 253(11): 1,390-1,394.
- Walker AL et al (2021). Association of diet with clinical outcomes in dogs with dilated cardiomyopathy and congestive heart failure, Journal of Veterinary Cardiology [Epub ahead of publication], DOI: 10.1016/j.jvc.2021.02.001.
- Ward J et al (2019). Association between atrial fibrillation and right-sided manifestations of congestive heart failure in dogs with degenerative mitral valve disease or dilated cardiomyopathy, Journal of Veterinary Cardiology 21: 18-27.
- Seckerdieck M et al (2015). Simpson’s method of discs in salukis and whippets: echocardiographic reference intervals for end-diastolic and end-systolic left ventricular volumes, Journal of Veterinary Cardiology 17(4): 271-281.
- Giraut S et al (2019). Breed-specific reference ranges for standard echocardiographic measurements in salukis, Journal of Small Animal Practice 60(6): 374-378.
- Esser LC et al (2020). Left ventricular M-mode prediction intervals in 7,651 dogs: population-wide and selected breed-specific values, Journal of Veterinary Internal Medicine 34(6): 2,242-2,252.
- Beier P et al (2015). The role of hypothyroidism in the etiology and progression of dilated cardiomyopathy in Doberman pinschers, Journal of Veterinary Internal Medicine 29(1): 141-149.
- Wesselowski S et al (2021). Prediction of clinically important acquired cardiac disease without an echocardiogram in large breed dogs using a combination of clinical, radiographic and electrocardiographic variables, Journal of Veterinary Cardiology [Epub ahead of publication], DOI: 10.1016/j.jvc.2021.07.003.
- Distl O et al (2007). Complex segregation analysis of dilated cardiomyopathy (DCM) in Irish wolfhounds, Heredity 99: 460-465.
- Simpson S et al (2016). Multiple genetic associations with Irish wolfhound dilated cardiomyopathy, BioMed Research International 2016: 6374082.
- O’Grady MR et al (2009). Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman pinschers, Journal of Veterinary Internal Medicine 23(5): 977-983.
- Schrope DP (2015). Prevalence of congenital heart disease in 76,301 mixed-breed dogs and 57,025 mixed-breed cats, Journal of Veterinary Cardiology 17(3): 192-202.
- Locatelli C et al (2013). Pulmonic stenosis in dogs: survival and risk factors in a retrospective cohort of patients, Journal of Small Animal Practice 54(9): 445-452.
- Oliveira P et al (2011). Retrospective review of congenital heart disease in 976 dogs, Journal of Veterinary Internal Medicine 25(3): 477-483.
- Ontiveros ES et al (2019). Congenital cardiac outflow tract abnormalities in dogs: prevalence and pattern of inheritance from 2008 to 2017, Frontiers in Veterinary Science 6: 52.
- Caivano D et al (2018). Murmur intensity in adult dogs with pulmonic and subaortic stenosis reflects disease severity, Journal of Small Animal Practice 59(3): 161-166.
- Bussadori C et al (2000). Guidelines for the echocardiographic studies of suspected subaortic and pulmonic stenosis, Journal of Veterinary Cardiology 2(2): 15-22.
- Kittleson MD and Kienle RD (1998). Pulmonic stenosis, Small Animal Cardiovascular Medicine, Mosby, St Louis: 248-259.
- Sosa I et al (2019). Stent angioplasty for treatment of canine valvular pulmonic stenosis, Journal of Veterinary Cardiology 21: 41-48.
- Patterson DF (1968). Epidemiologic and genetic studies of congenital heart disease in the dog, Circulation Research 23(2): 171-202.
- Singh MK et al (2011). Occlusion devices and approaches in canine patent ductus arteriosus: comparison of outcomes, Journal of Veterinary Internal Medicine 26(1): 85-92.
- Ramakrishnan S (2015). C3 – core curriculum in cardiology: Vascular plugs – a key companion to interventionists – ‘just plug it’, Indian Heart Journal 67(4): 399-405.
- Parag Barwad et al (2013). Amplatzer vascular plugs in congenital cardiovascular malformations, Annals of Pediatric Cardiology 6(2): 132-140.
- Saunders AB et al (2014). Long-term outcome in dogs with patent ductus arteriosus: 520 cases (1994-2009), Journal of Veterinary Internal Medicine 28(2): 401-410.
