18 Jun 2019
Heart disease patients: anaesthetic care and monitoring
Helen Benney details considerations around performing the vital perianaesthetic role required for patients with cardiovascular disease.

An RVN may frequently be required to provide the perianaesthetic care to patients with cardiovascular disease (CVD). These patients most commonly require general anaesthesia (GA) for routine surgical or diagnostic procedures not related to heart disease.
Alongside and under the direction of the vet, the RVN will be involved in the management and care of these patients in the perioperative period. They will then often be responsible for discharging the patient back to the owners’ care, ensuring they have all the information required for continuation of the animal’s recovery.
It is, therefore, important for the RVN to understand the basic haemodynamic changes of common CVD. By understanding the disease process, drug actions and the information monitoring equipment can provide, appropriate case management choices can be made to help promote a positive outcome.
Primary investigations
As with any GA, the aim is to provide unconsciousness, muscle relaxation and analgesia, while maintaining tissue perfusion and oxygen delivery. This becomes more challenging in patients with CVD (Robinson and Borgeat, 2016).
Most anaesthetic agents cause cardiovascular depression, and CVD reduces the ability to compensate for changes in heart rate, preload, afterload and cardiac output (CO), leading to an increased risk in perioperative mortality (Scarabelli and Bradbrook, 2016a).
All patients must be considered as an individual and have a protocol tailored to their specific needs, which becomes even more imperative when an underlying disease process exists.
The Association of Veterinary Anaesthetists (AVA) Guidelines for Safer Anaesthesia (Taylor et al, 2018) states: “Anaesthesia plan considered for each individual patient, covering patient risk factors, procedure risk factors, suitable anaesthesia drugs, fluids and monitoring aids.”
The owner should be questioned thoroughly in the preoperative assessment to establish the animal’s recent history – focusing on any changes in activity levels, appetite, changes in weight and any episodes of collapse. Exercise intolerance is an early sign of heart failure in many cases, as well as tachypnoea, dyspnoea, syncope or cyanosis. Details of current medication should be taken, as the effects of these drugs may be intensified by anaesthetic agents.
A thorough physical examination must be undertaken before planning the anaesthesia and the findings should be interpreted in the context of the individual patient. Stress from a clinical examination can change vital signs, so respiratory rate and effort – along with demeanour – is best observed from a distance first. Focus on the cardiovascular and respiratory systems may highlight issues that greatly impact the anaesthetic management, and auscultation should be carried out in conjunction with palpating a pulse to assess for any deficits.
It is important the RVN involved in the case is familiar with the clinical exam and understands details of the history taken. All information should be documented – this provides a “baseline” measurement, enabling changes in the perioperative period to become apparent sooner, recognised and acted on.
CVD will affect other organ systems, and this can impact on anaesthesia. Where CVD is suspected as a minimum of PCV and total protein, urea, creatinine and electrolytes, along with urine specific gravity (to assess glomerular filtration), blood pressure measurement and echocardiography should be performed (Robinson and Borgeat, 2016). Any anaemia should be corrected prior to GA to ensure the maintenance of an optimum oxygen-carrying capacity, cardiac insufficiency and anaemia will be poorly tolerated – and with a fixed CO tolerance is far less.
Echocardiography is the gold standard for assessing cardiac structure and function, but may not be available in all general practices. A referral for this prior to GA may be indicated for monitoring a current condition or for providing a diagnosis.
Radiographic diagnosis of CVD is based on size and shape of the cardiac silhouette, and on the pulmonary vessels, and is a useful tool. ECG for the evaluation of cardiac rhythm should be performed prior to GA when a conductance disturbance is suspected (Scarabelli and Bradbrook, 2016a).
Blood-based cardiac biomarkers can be tested for to assess myocardial injury. Natriuretic peptides and cardiac troponin represent blood-based substances that are associated with cardiac structure function and injury.
The decision to anaesthetise lies with the vet, and will be based on the degree and type of disease, along with clinical signs. The risks of developing chronic heart failure, poor CO and arrhythmias should be considered before undertaking GA in patients for elective procedures. Newly diagnosed or unstable patients with CVD should have GA for elective procedures postponed until stabilised.
Preanaesthetic care
Stress and pain activates the sympathetic nervous system and, therefore, increases cardiac workload; every effort should be made to avoid them where possible. An IV catheter is placed prior to anaesthesia for the administration of premedication, additional analgesia, induction agents, and any emergency drugs and fluids required.
To reduce any discomfort and stress, placement can be facilitated by preparing the skin over the vein by applying a local anaesthetic cream. If time is limited, a skin cooling spray can be applied. Premedication is reasonable to reduce the level of stress, minimising tachycardia, catecholamine release and myocardial work (Scarabelli and Bradbrook, 2016b).
Preoxygenation prior to induction of anaesthesia is important to delay the desaturation that may occur post-induction; 100% oxygen provided via a tight-fitting face mask will provide the most benefit, but is rarely tolerated and may induce more stress. In this case, holding the O2 source close to the nares will provide some benefit and may be better tolerated if the flow is directed across, rather than towards, the nares.
Body temperature should be monitored closely perioperatively. Hyperthermia, due to stress or overactive warming of the patient, may cause an increase in metabolic rate and an increase in oxygen consumption, which may result in hypoxia. Hypothermia can again cause an increase in oxygen consumption due to shivering, as well as prolonged recoveries, decreased anaesthetic agent requirements and ECG changes.
Anaesthesia
With cardiac compromise, aims during GA include:
- maintaining CO
- maintaining blood pressure within normal limits
- avoiding excessive changes in heart rate (tachycardia/bradycardia)
- maintaining cardiac contractility
- maintaining adequate ventilation
- avoiding fluid overload, as well as underload (Dugdale, 2010).
See Table 1 for specific condition considerations.
| Table 1. Common cardiovascular pathology and the anaesthetic considerations | ||||
|---|---|---|---|---|
| Mitral valve disease | Dilated cardiomyopathy | Hypertrophic cardiomyopathy | Subaortic and pulmonary stenosis | |
| Pathology | Valve prolapse and insufficiency. Blood flows back into the left atrium from the ventricle rather than leaving the heart via the aorta. Resulting decrease in stroke volume. |
Reduced systolic function (reduction in cardiac output; CO), decreased myocardial contractility. Volume overload and dilation of the left ventricle (LV). Common complications are arrhythmias, ventricular premature complexes or atrial fibrillation. |
Hypertrophy of the LV, leads to stiffness, the ventricle cannot relax as it should (diastolic dysfunction) or contract. There is a reduction in CO. Increasing myocardial oxygen demand with no increase in coronary perfusion, leads to myocardial hypoxia and promotes arrhythmias. |
Aortic stenosis is classified depending on the location of the obstruction and most commonly occurs in the subaortic region. Stroke volume limited leading to LV pressure overload. This pressure increases myocardial O2 demand and ischaemia. Valvular stenosis is the most common form of pulmonary stenosis. |
| Anaesthetic considerations | Maintain heart rate, preload and contractility, while avoiding an increase in afterload. |
Cardiac output dependent on heart rate, so large changes should be avoided. Maintain preload/venous return. Myocardial contractility should be maintained or increased. |
May have a genetic origin or can be secondary to hyperthyroidism or chronic kidney disease. High heart rates (stress) should be avoided. A normal or slightly lower heart rate must be maintained to enhance diastolic filling time. A mild reduction of contractility will decrease myocardial oxygen consumption. |
Similar for both. CO is dependent on heart rate. Stroke volume cannot be increased due to the outflow obstruction. Atrial contraction is fundamental for ventricular filing. Vasodilation will reduce systemic blood pressure worsening preload, adequate venous return is required to fill the ventricle. Maintaining normal heart rate will limit myocardial O2 deficit in the case of tachycardia. |
Anaesthetic drugs are selected on the basis they minimally aggravate the existing condition, and it is important an understanding exists of drug behaviour and potential interactions (White, 2012).
No one protocol exists for all patients with CVD as the pathophysiology of each disorder is different. If not contraindicated, using drugs familiar to the vet and RVN is important, and emergency drug dose calculations should be pre-calculated and readily available to treat any potential problems. Table 2 shows some examples of specific CVD, and anaesthetic and analgesic drugs to consider.
| Table 2. Cardiovascular diseases and drug considerations | |||||
|---|---|---|---|---|---|
| Mitral valve disease | Dilated cardiomyopathy | Hypertrophic cardiomyopathy | Subaortic stenosis | Pulmonary stenosis | |
| Premedication | Acepromazine (low dose), benzodiazepines, opioids | Acepromazine (low dose), benzodiazepines, opioids | Alpha-2 agonists, opioids | Opioids, benzodiazepines | Opioids, benzodiazepines |
| Induction | Alfaxalone, propofol | Opioids, benzodiazepines, alfaxalone, propofol | Propofol, alfaxalone | (Etomidate), ketamine/benzodiazepine, propofol, alfaxalone (slow to effect to minimise effects on systemic vascular resistance; SVR) |
(Etomidate), ketamine/benzodiazepine, propofol, alfaxalone (slow to effect to minimise effects on SVR) |
| Maintenance | Isoflurane, sevoflurane | Isoflurane, sevoflurane | Isoflurane, sevoflurane (off licence in cats) | Isoflurane, sevoflurane | Isoflurane, sevoflurane |
| Analgesia (and for anaesthetic sparing effects) | Opioid constant rate infusion (CRI), lidocaine CRI, nerve blocks | Opioid CRI, lidocaine CRI, ketamine CRI, nerve block, epidural morphine | Epidural morphine, opioid CRI | Epidurial morphine, opioid CRI | Lidocaine CRI, opioid CRI (avoid bradycardia) |
| Additional drug therapy as required | Positive inotropes (hypotension), anticholinergenics (bradycardia) | Positive inotropes (hypotension), lidocaine (arrhythmias) | Vasopressors (hypotension) | Vasopressors (hypotension), anticholinergenics (bradycardia) | Vasopressors (hypotension) |
| Fluid therapy | Care with volume overload | Care with volume overload | Prevent hypovolaemia | ||
| Contraindicated | Alpha-2 agonists, acepromazine (if on angiotensin-converting enzyme inhibitors), ketamine (where arrhythmias or tachycardia present) |
Alpha-2 agonists, epidural local anaesthetics | Ketamine, positive inotropes | Alpha-2 agonists, acepromazine, epidural local anaesthetics | Alpha-2 agonists, acepromazine, epidural local anaesthetics |
| Scarabelli and Bradbrook, 2016b; Curtic-Uhle and Waddle, 2010; Robinson and Borgeat, 2016. | |||||
Systemic analgesia and local anaesthetic techniques (multimodal analgesia) should be implemented as part of a well-balanced anaesthetic technique to reduce the amount of induction and inhalant drugs required to maintain GA, reducing their negative side effects on the cardiovascular system (Clifforde, 2018). These include nerve blocks, local infiltration and splash blocks, constant rate infusions of suitable analgesics and NSAIDs.
Monitoring postoperatively
Recovery is a high-risk time of anaesthesia. In the Brodbelt et al (2008) study, 61% of cat and 47% of dog anaesthetic-related deaths were found to be in the postoperative period. Patients should be receiving one-to-one care from the RVN until extubating and should be under constant observation until they are alert.
Some clinical signs of heart failure include increased respiratory rate, dyspnoea, crackles or muffled lung sounds. Pale mucous membranes and a prolonged capillary refill time – reflective of peripheral vasoconstriction – can be an indirect indication of reduced CO or “forward” failure, along with cold extremities in cases of severe heart failure (Cobb, 2014).
Temperature, respiration rate/effort, heart rate, pulse quality, mucous membrane colour and capillary refill time should all be monitored and documented during the recovery period. It may be of benefit to provide flow by O2 to these patients in the immediate recovery period to improve myocardial oxygenation, and if any pulse deficits noted, an ECG placed.
Pain scores should be carried out on a regular basis using a validated pain scoring system (such as the Glasgow Composite Pain Scale). The AVA Guidelines for Safer Anaesthesia (Taylor et al, 2018) state: “An analgesic plan should be made for each case recognising the expected level and modality of pain. Patients should be actively assessed using validated pain scores and results responded to appropriately.”
Pain has many detrimental effects on the body – one of them being activation of the stress response as aforementioned. This increases cardiac workload, exacerbates arrythmias and impairs ventilation.
Monitoring the cardiovascular system
Although a trained and focused anaesthetist is the most vital instrument for safely monitoring an anaesthetic, and cannot be replaced by a machine, the additional information gained by multiparameter monitoring cannot be objectively gained by a person alone (Taylor et al, 2018).
By collecting and interpreting and understanding the information a more detailed picture of the patient status can be gained and case management improved.
The aim of monitoring the cardiovascular system is to assess the delivery of oxygenated blood to vital tissues. Oxygen delivery to tissues is determined by the oxygen-carrying capacity of the blood and CO (Panel 1). If CO is reduced due to CVD then both O2 delivery to tissues and removal of waste products is decreased (Scarabelli and Bradbrook, 2016a). Monitoring the cardiovascular system allows factors contributing to O2 delivery to be optimised (Self, 2015).
- Oxygen delivery = cardiac output (CO) × arterial oxygen content
- CO = stroke volume (SV) × heart rate>
- Mean arterial pressure = CO × systemic vascular resistance
- SV = volume of blood ejected per contraction, dependent on cardiac contractility, preload and afterload
As CO can be expressed as the product between mean arterial blood pressure (MAP) and systemic vascular resistance, monitoring MAP can give us an estimation of CO. Hypotension is a common complication in anaesthetised animals yet only monitored in 11% of dogs and 10% of cats anaesthetised in general practice (Filipas and Vettorato, 2018).
Doppler (commonly available in most practices) measures systolic arterial pressure (SAP) only; in normotensive dogs it has shown acceptable correlation, but accuracy drops during hypotension/hypertension.
In cats, Doppler has been shown to underestimate SAP by up to 25mmHg, but correlates well with MAP (Self, 2015). The oscillometric method will provide the systolic, mean and diastolic readings; it may be less reliable in cats and small dogs. Using the correct cuff size of 40% circumference of limb and level with the right atrium is important for accuracy of these two methods.
With both these methods, obtaining a pre-GA value and monitoring trends is useful. Invasive blood pressure (IBP) is considered the gold standard as it provides continuous monitoring and display of a pressure waveform, and has greater reliability. To measure IBP, a catheter is placed in a peripheral artery (most commonly the dorsal pedal artery in cats and dogs) and connected via a transducer to the multiparameter monitor. Changes in contractility, vasodilation and vasoconstriction will alter the shape and amplitude of the waveform (Scarabelli and Bradbrook, 2016b).
Until recently, it has not been possible to monitor CO due to the invasiveness of most techniques, their high costs and specialist skills required. The thermodilution technique most commonly used in a research setting and considered gold standard requires a pulmonary artery catheter (PAC) to be placed.
One newer method now available to veterinary practice is the pressure recording analytical method (PRAM) MostCare (Figure 1). This is a pulse contour method that estimates stroke volume and other haemodynamic parameters from the analysis of the arterial pulse waveform, and reads this from the peripheral arterial line placed for IBP monitoring.
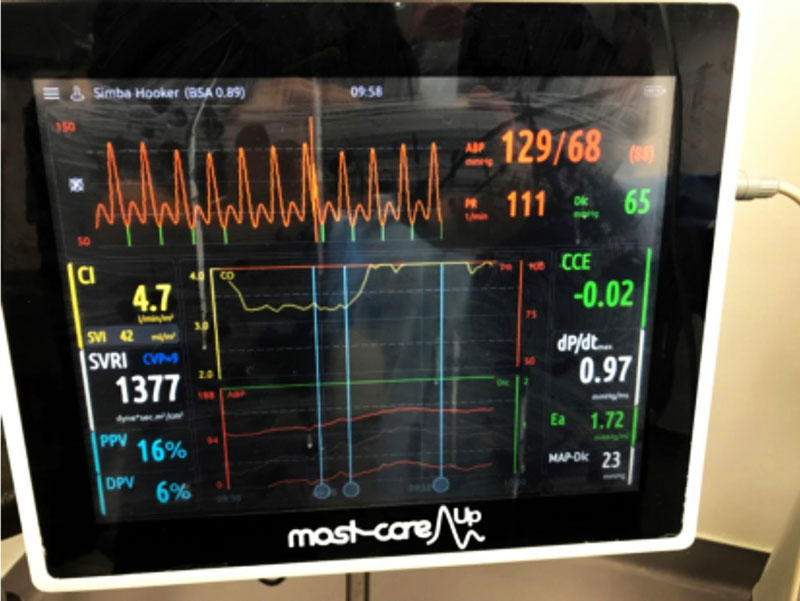
A preliminary study by Briganti et al (2018) concluded PRAM resulted in good precision for the measure of CO in the anaesthetised dog, and represented a promising alternative to thermodilution.
Pulse oximetry provides information on the oxygen saturation of haemoglobin in arterial blood (SpO2). Hypoxaemia is normally defined as PaO2 less than 60mmHg, which corresponds to a SpO2 less than 90%. For a reliable reading the plethysmogram (signal waveform) should closely resemble an arterial pressure wave form, and the pulse rate displayed be correct.
If not, the SpO2 reading may be considered unreliable. Factors such as poor perfusion, movement, light and electrosurgical equipment inference may all affect readings. Many pulse oximeters now provide a perfusion index reading, indicating tissue perfusion levels and therefore indicating reading reliability.
In anaesthetised mechanically ventilated patients, systolic pressure variability (SPV), pulse pressure variability (PPV) and plethysmograph variability index (PVI) can predict fluids responsiveness to hypotension. The cyclic effect of intrathoracic pressure changes can be visualised as a “swing” in the IBP wave form or pulse oximeter (Figure 2).
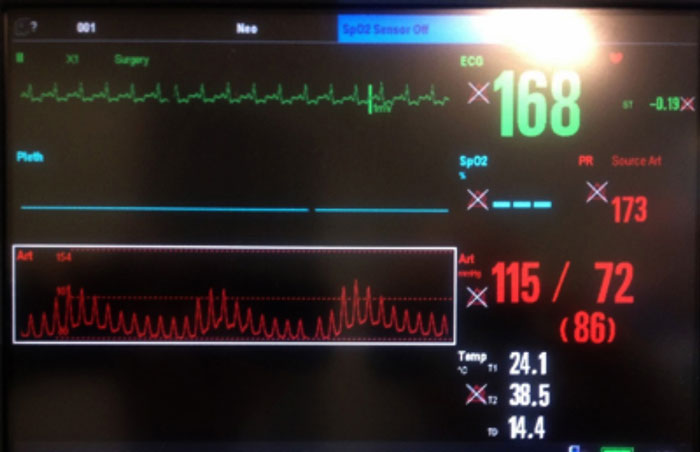
A PPV of greater than or equal to 13%, PVI of greater than or equal to 14% or SPV of greater than or equal to 4.5% can be used in dogs to detect hypovolaemia and predict fluids’ responsiveness. The mini bolus technique (2ml/kg to 3ml/kg over one minute) provides minimal risk of fluid overload in patients with CVD (Filipas and Vettorato, 2018).
ECG is used for monitoring the electrical activity of the heart, and as some CVD and drugs used can predispose to arrhythmias during GA, ECG becomes an important modality in these cases. The VN should be familiar with the normal complex and potential common arrhythmias, enabling him or her to alert the vet of any changes for a diagnosis and treatment.
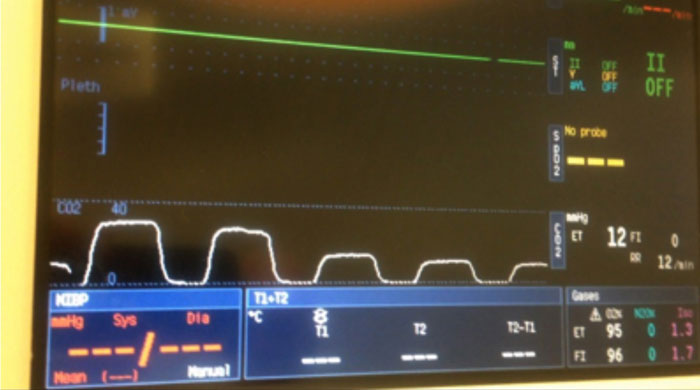
Capnography provides real-time information on respiratory rate, end-tidal CO2 and inspired CO2 levels. The wave form on the capnogram will provide useful information on respiratory rate, as well as information regarding leaks in the breathing system, resistance to breathing and rebreathing of CO2. As well as information on ventilation, the capnograph can provide an indication of CO.
With a consistent respiratory rate and tidal volume (such as when ventilated), a sudden reduction in CO will result in the concentration of expiatory CO2 reducing over several breaths. This is due to CO2 being produced by the tissues no longer being transported to the lungs, and will be seen in cardiac arrest, pulmonary embolism and major reductions in CO (Figure 3).
Conclusion
Anaesthetising patients with CVD presents an increased risk, so planning and preparation for the individual case is important. The vet will select a drug protocol with minimal effects on the cardiovascular system and suitable for the disease process, and the RVN will be required to understand the physiological effects of the disease and how GA will influence these.
By suitably monitoring the patient throughout the hospitalisation and GA periods, the vet can be alerted to any potential concerns rapidly. The entire hospitalisation period should be maintained as stress free as possible, including provision of suitable analgesia and a comfortable environment with minimal noise. Provision of supplemental O2 both pre-GA and post-GA is warranted and maintaining patent IV access throughout is vital.
- Some drugs mentioned in this article are used under the cascade.
- Reviewed by Chiara De Gennaro DVM, DipECVAA, MRCVS.
