13 Jan 2025
Human toxocariasis control
Ian Wright BVMS, BSc, MSc, MRCVS covers the controversies and practicalities of keeping pets and owners safe from this disease.
Toxocara species is a group of intestinal nematodes with species infecting dogs (T canis) and cats (T cati). It is the cause of human toxocarosis, a significant zoonosis that can lead to visceral, ocular and neurological larval migran,s as well as the condition known as covert toxocarosis.
The ocular form of the disease has a relatively high public profile, and toxocarosis remains an enigmatic, controversial and emotive disease which continues to debilitate members of the public.
Some aspects of toxocarosis control are very clear, while others are more controversial. It is the task of the veterinary professional to weigh the evidence surrounding Toxocara species transmission and disease risk, and then give clear recommendations to help keep pets and the pet-owning public safe.
Few topics in the field of veterinary public health are as emotive as human toxocariasis. Pictures of blinded children in the newspapers lead to accusing eyes upon dogs in parks and communal areas, and the fear that contact with dogs may lead to debilitating illness.
Conversely, many dog owners and pro-dog societies consider toxocariasis as the stick anti-dog lobbyists use to beat them with and to reduce their freedom to roam where they choose.
Within the veterinary profession, Toxocara species control forms the basis of deworming frequency advice alongside lungworm and tapeworm control.
Large knowledge gaps are present in the extent to which the UK population is exposed to Toxocara species, and it is the task of the veterinary professional to weigh the evidence we have and give clear recommendations to keep the pet-owning public safe.
Life cycle and transmission in cats and dogs
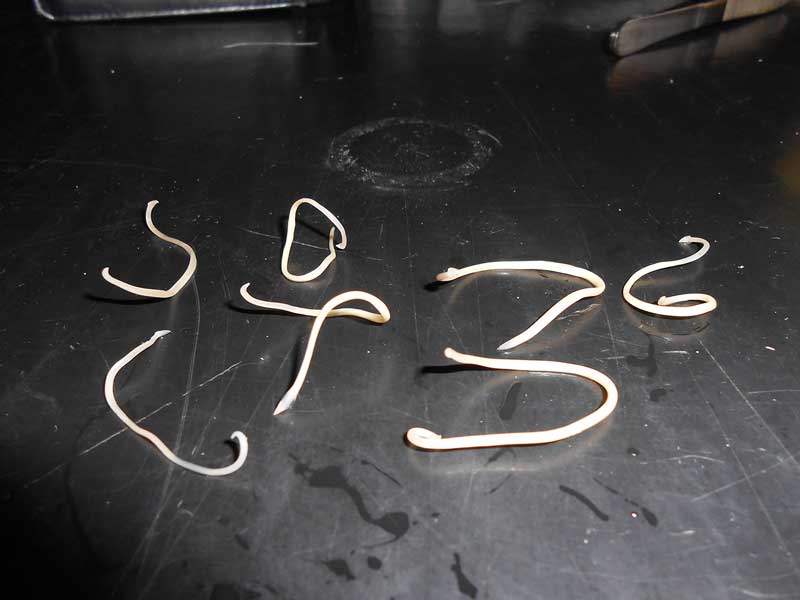
The life cycle for T canis is complex. Adult worms (Figure 1) live in the small intestine and shed eggs into the environment via the faeces of the host. The eggs when first shed are unembryonated (Figure 2) and are not infective.
Progression to the infective embryonated L3 stage is required for infection, so fresh faeces do not present a zoonotic risk.
Embryonation takes place in two to seven weeks under optimum conditions. Dogs may be infected by ingesting embryonated eggs, consuming paratenic hosts or via transplacental and transmammary routes.
Transplacental infection means that the prevalence of infection in pups born to untreated dams can be very high. Transmammary infection or consuming paratenic hosts, such as rodents, is more important in T cati transmission where the feline host frequently hunts, and transplacental transmission does not occur. Transmammary infection ensures high prevalence in puppies and kittens that have not had anthelmintic treatment.
As cats and dogs age, they develop a degree of immunity to the parasite, and prevalence of patent infection is highly variable, with one UK study having found 5% of dogs and 26% of dogs to be shedding ova (Wright et al, 2016).
Eggs are long lived in the environment, and cats and dogs may shed intermittently, leading to build up of eggs over time. Studies of parks in England have shown high levels of egg contamination, representing significant zoonotic risk through egg ingestion (Airs et al, 2022).
Visceral larval migrans. Migrating larvae can lodge in the lungs or liver, leading to respiratory signs and/or hepatomegaly associated with a high eosinophilia. Other organs may be affected, such as the heart, leading to myocarditis. Fever, weight loss, abdominal pain, anorexia and lethargy are all common in diagnosed patients, with the potential for respiratory and cardiac signs.
Ocular larval migrans. Perhaps the most well-known consequence of infection. Migrating larvae in the eye set up granulomatous reactions. Associated endophthalmitis, chorioretinitis, uveitis and retinal detachment can then occur, leading to progressive visual loss, strabismus and blepharospasm.
Neurological larval migrans. Migrating larvae in the nervous system can lead to infarction and meningoencephalitis. Behavioural changes, changes in sleep patterns and seizures have all been linked to Toxocara species infection.
Covert toxocariasis. Seropositivity can be associated with a mild or moderate eosinophilia, and associated mild multiple clinical signs that may or may not be due to migrating larvae. Abdominal pain, headache, cough, lethargy and a rash, with or without pruritus, have all been reported in eosinophilic, seropositive patients.
Being seropositive for Toxocara species has also been shown to have associations with increased risks of chronic conditions, such as asthma, epilepsy and cognitive impairment (Overgaauw and van Knapen, 2013).
Although people can be infected by eating the undercooked meat of paratenic hosts such as wild game, the most common route of human infection is by the ingestion of embryonated eggs. This can occur through the accidental or deliberate consumption of soil (geophagia), the transfer of the sticky eggs on to other objects that may be placed in the mouth (pica), or, less commonly, through contact with contaminated dog hair (direct dog contact).
It is, therefore, desirable to limit exposure to infection.
However, a number of controversies surround human toxocariasis and its control.
Dog access to public spaces
Banning dogs from children’s playgrounds and sports fields has long been a controversial issue, and is often debated in the media. These debates quickly become highly emotive, with loud voices on either side of the dog lobby.
Public park spaces are difficult to ban dogs from, as many dog owners (including the author) enjoy exercising dogs there. Dog fouling is an issue, with perceived levels of dog fouling linked to levels of egg contamination in public spaces (Airs et al, 2022).
Even if dog fouling events are uncommon, over time, ova levels may build up in playgrounds and sports fields, making it difficult to justify not banning dogs from these areas. Where dogs have access, promoting responsible disposal of dog faeces is vital to help prevent transmission of a range of pathogens.
Role of cats
It was originally thought that T canis alone was the source of human infection by this route, but strong evidence now exists to suggest that T cati is significantly involved, too.
Although some still consider the role of T cati in human toxocariasis to be controversial, the large number of common antigenic fractions between T canis and T cati, and the similarity in mode of transmission, would suggest that the two parasites have comparable zoonotic risk (Cardillo et al, 2009).
The potential role of T cati in human toxocariasis, therefore, cannot be ignored or underestimated. Although recent park studies have found T canis eggs to be predominating, cats often defecate in niche areas such as sandpits, allotments and kitchen gardens, increasing the risk of human exposure.
Clients should be advised to cover sandpits when not in use, to prevent cats defecating in them and burying their faeces.
Cats should be treated for roundworm in a similar fashion to dogs to reduce environmental contamination.
Role of foxes
Although prevalence of T canis in foxes is high and their role in environmental contamination has not been quantified, it is thought to be less than 10% of dogs. This is due to the far fewer number of foxes than dogs found in most populated areas. Therefore, although keeping foxes out of children’s playgrounds and parks with fencing is sensible if possible, it is unlikely that fox population control would have any significant impact on human toxocariasis incidence.
Direct dog contact
A number of studies have found embryonated Toxocara species eggs in the coats of dogs (Wolfe and Wright, 2003; Roddie et al, 2008), suggesting that transmission may occur through direct contact with the coat of dogs.
It has also been demonstrated, however, that T canis eggs embryonate in dog fur at a much lower rate than in soil (Keegan and Holland, 2013).
Although it is unlikely that embryonated Toxocara species eggs in fur do not pose as great a risk as those in soil, good hand hygiene will further reduce any risk this route poses and should be strongly encouraged – especially in young children. This will also help to prevent the transmission of other faecal-oral parasites such as Campylobacter species, Giardia species and Toxoplasma gondii.
Regular deworming in the face of low human case rates in the UK
Debate surrounds the effectiveness of deworming four times a year as a means of limiting Toxocara species egg shedding over time. The minimum effective deworming frequency is uncertain and further research is needed – particularly focusing on longitudinal studies.
In the meantime, the European Scientific Counsel Companion Animal Parasites recommends deworming cats and dogs with outdoor access at least four times a year, based on studies that have modelled or demonstrated efficacy at this frequency (Nijsse et al, 2015; Wright and Wolfe, 2007). Use of an effective monthly anthelmintic will reduce shedding further and is recommended in cases where risk of pet exposure is increased, or where pets are living with groups at higher risk of disease.
The counter argument is that regular deworming may lead to environmental contamination with anthelmintics or encourage resistance to anthelmintics. The risk of resistance, however, is only reduced if parasites not exposed to anthelmintics are released into the environment to create refugia. This is not achieved by not treating pets negative for parasites. Allowing some shedding of ova into the environment to limit resistance is hard to justify for those at risk of significant zoonoses such as toxocariasis.
No evidence exists of environmental contamination with anthelmintics originating from products intended for use in cats and dogs. Further research is required to begin to quantify this risk.
Concerns regarding over treatment, the development of anthelmintic resistance and environmental contamination have led to the question of whether regular testing of pets could replace routine preventive treatment for intestinal roundworms in cats and dogs. This is a viable option, but would need to be performed at least quarterly to avoid prolonged human exposure to zoonotic life stages.
Routine testing alongside treatment for intestinal roundworms is of benefit to practices both in terms of reinforcing good practice among pet owners, identifying which parasites are present in a local area and early detection of drug resistance. It can be performed at annual or six-monthly heath checks.
Preventing human toxocariasis and role of treatment and testing
A number of steps can be taken to reduce the impact on pets’ health caused by worms and minimise zoonotic risk.
- Treatment of adult cats with outdoor access and dogs with a licensed anthelmintic at least every three months to reduce Toxocara species egg shedding, or faecal testing at a similar frequency. If the latter approach is adopted, owners should be made aware that shedding can occur between tests.
- Monthly treatment to eliminate most egg shedding, recommended for:
- Cats or dogs fed raw, unprocessed diets or those that hunt or scavenge.
- Puppies and kittens less than six months of age.
- When the animal is in regular contact with children or immunocompromised individuals.
- Regular picking up and responsible disposal of dog faeces to reduce environmental contamination.
- Excluding pets from children’s play areas.
- Covering of sand pits when not in use.
- Thorough washing of fruit and vegetables prior to consumption.
- Good hand hygiene to limit transmission via hand to mouth contact.
Future research and conclusions
Despite extensive research, much is still unknown about toxocariasis and further research is needed.
This includes how large a role cats play in transmission, the effectiveness of four-times-a-year deworming regimes, the seroprevalence and incidence of disease in the UK population and the link between the two. Little or no data exists on environmental contamination with anthelmintic from pet products and, here also, further research is needed.
In the meantime, we do know that environmental contamination with Toxocara species eggs is considerable, and the UK public is being exposed to a preventable zoonotic pathogen.
Veterinary professionals have a vital role to play in advising pet owners on deworming and faecal testing of pets, alongside promoting anti-dog fouling campaigns.
- Vet Times, Volume 54, Issue 51, Pages 6-8

