26 Jun 2017
Fiona Adam and James Histed describe methods of assessing high blood pressure in felines, causes of the condition and various organ damage.
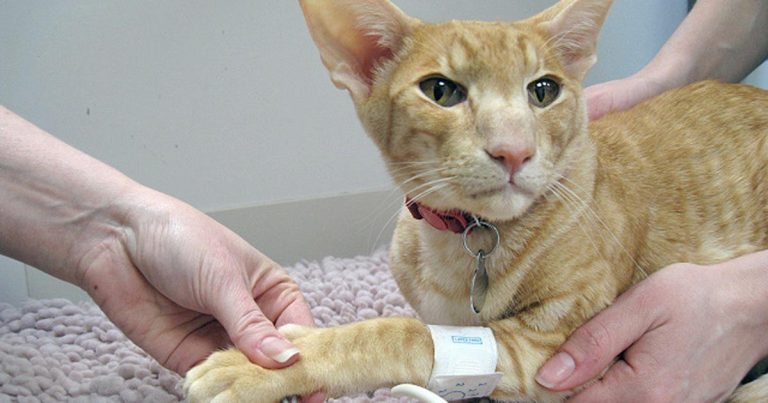
Figure 1. Performing a Doppler blood pressure assessment in a cat.
Hypertension can cause catastrophic, potentially irreversible, damage to organs such as the eyes, brain, heart and kidneys.
The prevalence of hypertension, and the underlying disease states that predispose to it (Table 1), increase with age. Blood pressure (BP) assessment is, therefore, recommended as part of routine health screening in senior cats and also as part of the ongoing management in cats diagnosed with diseases known to predispose to hypertension (Table 2).
| Table 1. Diseases/medications known to predispose to hypertension | |
|---|---|
| Disease/medication | Reported prevalence |
| Chronic kidney disease (CKD) | 20% to 65%. Neither the likelihood of hypertension nor its severity is related to the stage of CKD. Hypertension can develop in cats with CKD prior to the development of azotaemia. |
| Hyperthyroidism | 10% to 25%. It is important to note some hyperthyroid cats that are normotensive at presentation develop hypertension at a later stage, despite successful control of their hyperthyroidism. |
| Primary hyperaldosteronism (Conn’s syndrome) | 50% to 100%. |
| Diabetes mellitus | Hypertension in cats with diabetes is very rare. |
| Phaeochromocytoma | Rare condition in cats, thus prevalence of hypertension with this diagnosis is unknown. In dogs, around 50% to 85% of phaeochromocytoma patients are hypertensive. |
| Erythropoietin/darbepoietin treatment | 25% to 50%. |
| Table 2. When to assess blood pressure in cats | ||
|---|---|---|
| Category | Frequency of systolic blood pressure monitoring | Rationale for recommendation |
| Healthy adult cats (three to six years old) | Consider every 12 months | Hypertension in this age group should be very rare, with the exception of those cats with known predisposing medical conditions, such as chronic kidney disease. However, establishing baseline values for an individual cat, for reasons of comparison in later life, could be useful. Interpretation of elevated blood pressure values, particularly in the absence of target organ damage (TOD), should be made with great care, given the low frequency of hypertension in this age group and the possibility of white coat hypertension. |
| Healthy senior cats (more than seven years old) | At least every 12 months | Prevalence of hypertension increases with age and early detection can prevent TOD and aid detection of important underlying medical conditions, such as hyperthyroidism. |
| Cats with recognised medical conditions predisposing to hypertension | Measure at diagnosis and reassess at least every three to six months, depending on the stability of the underlying condition | Hypertension can develop as a complicating factor in many medical conditions and alongside some pharmaceuticals (for example, erythropoietin). |
| Cats with clinical findings potentially compatible with TOD secondary to hypertension | Measure at diagnosis | TOD can develop secondary to hypertension in the heart, eyes, kidneys and brain. Blood pressure assessment should be considered an important early part of diagnostic investigations. |
| Recommendations taken from the International Society of Feline Medicine consensus guidelines on the diagnosis and management of hypertension in cats (Taylor et al, 2017). | ||
In addition to idiopathic hypertension and hypertension secondary to underlying diseases, it is important to consider the impact stress has on BP. (Panel 1). White coat hypertension is an important cause of elevated BP readings in the clinic setting and can potentially lead to a false diagnosis of systemic hypertension.
White coat hypertension is a term used to describe increased blood pressure seen during patient anxiety at a medical facility.
It occurs as a result of sympathetic nervous system stimulation. The typical magnitude of systolic blood pressure elevation is around 20mmHg, but huge variation has been seen with elevations of 80mmHg recorded (Belew et al, 1999). Unfortunately, the presence of tachycardia in these patients is not a reliable indicator of white coat hypertension and some cats exhibiting marked white coat hypertension may have normal heart rates.
In human medicine, 24-hour ambulatory devices are often used to monitor blood pressure in the patient’s home environment to minimise the complicating factor of white coat hypertension. Unfortunately, this is not a practicable option in small animal practice. Attempts to minimise the stress associated with veterinary visits and the blood pressure measuring procedure are vital to try to minimise the likelihood of a patient’s blood pressure readings being affected by white coat hypertension.
When assessing BP, in human medicine both systolic blood pressure (SBP) and diastolic blood pressure (DBP) are considered, but in small animal practice SBP receives the focus. In part, this relates to challenges associated with obtaining accurate DBP measurements and, in part, is due to evidence in other species SBP is a more important determinant of hypertension-associated tissue damage (Brown et al, 2007).
SBP in healthy cats is typically 110mmHg to 130mmHg. Hypertension has been defined and stratified on the basis of the risk of target organ damage (TOD; Table 3). It is, however, important to note the risk of hypertension-associated tissue damage is likely to be influenced by the rate of development of hypertension and its duration, as well as the actual BP values.
| Table 3. Relationship between systolic blood pressure and target organ damage risk | ||
|---|---|---|
| Systolic blood pressure | Category | Risk of target organ damage |
| Below 150mmHg | Normotension | Minimal |
| 150mmHg to 159mmHg | Borderline hypertension | Mild |
| 160mmHg to 179mmHg | Hypertension | Moderate |
| Equal to or more than 180mmHg | Severe hypertension | Severe |
| Taken from the American College of Veterinary Internal Medicine consensus statement: Guidelines for the identification, evaluation and management of systemic hypertension in dogs and cats (Brown et al, 2007). | ||
BP can be assessed directly by arterial cannulation or indirectly with techniques employing a compressive cuff. Intra-arterial, direct BP assessment techniques enable accurate determination of SBP, DBP and mean arterial pressures (MAP). However, due to the practicalities of this technique, it tends to be restricted to use in anaesthetised patients.
In clinical practice, BP assessment is more commonly performed by indirect means – doppler sphygmomanometry and oscillometry. Both of these techniques are based on the use of a compressive cuff to occlude arterial flow and measure the BP as vascular flow returns.
A compressive cuff is inflated to occlude arterial flow and the pressure slowly released while an ultrasound probe over a distal artery awaits the return of blood flow. The pressure in the cuff at the point when arterial flow is heard to return is considered to be the patient’s SBP. DBP is typically not determined using this technique as values do not correlate well with direct measures.
Oscillometers are available as sole instruments or are often built into multiparameter monitors (typically used during anaesthesia). The cuff is placed on the patient and the machine activated. The cuff is inflated and, during deflation, the oscillometer measures alterations in the cuff pressure generated by pulse pressure changes. The largest amplitude of pressure change in the cuff equates to the MAP. The SBP equates to the pressure when oscillations in the cuff are first detected, and the DBP is the pressure when oscillations rapidly reduce.
In practice, the SBP and DBP are both calculated by the oscillometer from the MAP using built-in algorithms. Oscillometers are considered less accurate in small patients or when arrhythmias are present. It is important to check the pulse rate being reported by the oscillometer is correct, as an erroneous pulse rate is likely to be associated with inaccurate BP assessment.
High-definition oscillometry (HDO) addresses some of the problems of traditional oscillometry. HDO performs real-time analysis of arterial wall oscillations to obtain pressure wave amplitudes, allowing both SBP and DBP to be measured directly, rather than calculated from the MAP reading. Even with HDO, however, MAP and DBP do not correlate well with directly measured values, and only the SBP is considered accurate (Martel et al, 2013). HDO should allow accurate BP assessment even in the presence of marked tachycardia or arrhythmias.
To minimise the possibility of white coat hypertension, BP assessment should be performed in a calm and quiet environment that the patient has had time to acclimatise to (Figure 1). Ensure the patient is settled and comfortable; cats will often settle well in the base of their carriers on a blanket. If restraint is required, this should be done calmly and gently. Chemical restraint should be avoided as it can affect the BP.
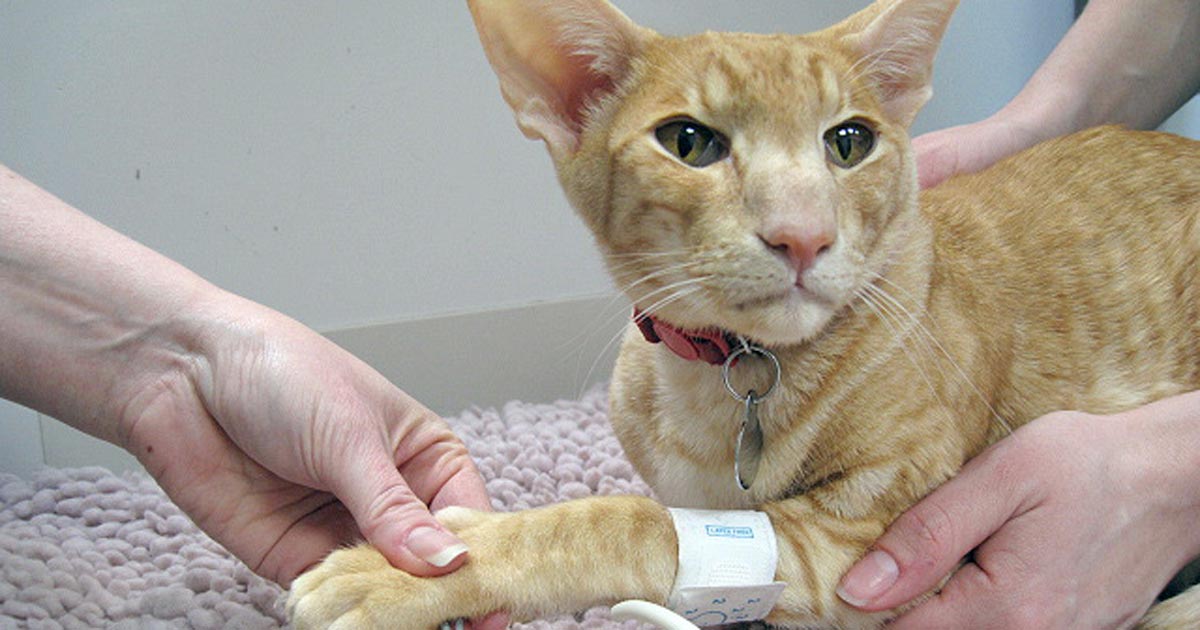
Trained, experienced personnel have been shown to achieve more reproducible BP readings (Gouni et al, 2015). In many practices, nurses and technicians are very accomplished at obtaining BP measurements. If the owner is thought to be a calming influence, he or she can be a useful presence and may help settle the cat. Checking a cat’s BP while it is resting on its owner’s knee is a great option.
The cuff should be applied on the limb or tail. The location used will most likely be determined by the cat’s position and its tolerance of the cuff at the site. Variation is found between the readings obtained at each site, so it is advised the site you have used (and the cuff size) is recorded to ensure future BP assessments are made with the same variables.
The cuff size (for example, five) indicates the width of the cuff in centimetres. The cuff size should equate to 40% of the circumference of the site where the cuff is to be placed (Figure 2). Too large a cuff will result in erroneously low BP readings and a cuff too small will result in falsely elevated readings. Most cuffs close with Velcro, which can loosen and pop open during inflation. A small length of tape can be used to prevent the cuff opening during inflation, but be sure not to wrap the tape entirely around the cuff, as this may alter pressures during inflation and invalidate the BP readings obtained.
The cuff should be situated at the height of the right atrium. A cuff substantially lower than this point will result in falsely elevated BP readings. If the patient is in lateral recumbency, the sternum can be used approximately to be the height of the right atrium. In sternal recumbency, the right atrium is located around 40% up an imaginary line between the sternum and caudal border of the scapula.
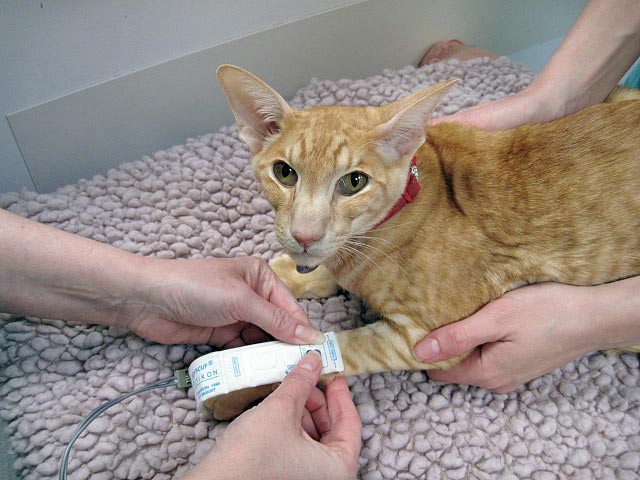
If using the Doppler technique, the ultrasound probe needs good contact to determine the arterial flow. In many feline patients, simply wetting the hair with gel will allow adequate probe contact. If clipping is required, using quiet cat clippers is ideal to minimise stress. The whooshing sound of the arterial pulsation detected by the Doppler can startle some patients and you may want to consider using headphones when performing this technique.
Multiple readings should be obtained to try to ensure an accurate representation of SBP is being achieved. The first reading should be discarded and a further three to seven readings taken, which should show less than 20% variability. If the readings vary more than this, further measurements, or reassessment a short while later, would be advised.
Once three to seven consistent readings have been obtained consecutively, they should be averaged for the SBP measurement.
When a hypertensive BP reading is obtained, this result could be due to genuine hypertension or white coat hypertension. Erroneous diagnosis of white coat hypertension as genuine hypertension would result in unnecessary administration of antihypertensive medication.
The presence of TOD can assist in determining hypertensive BP readings are likely genuine, rather than related to white coat hypertension. When faced with elevated SBP measurements, assessment for evidence of TOD can be very useful (Panel 2). TOD may, of course, be the first finding that facilitates the diagnosis of hypertension too, such as an elderly cat presenting with blindness due to bilateral retinal detachments.
The eyes, brain and kidneys are vulnerable to hypertensive damage due to their extensive arterial supply. The heart is vulnerable to hypertrophy in hypertension as a result of the increased workload associated with increased systemic vascular resistance.
Although SBPs less than 150mmHg are considered to be associated with a minimal risk of TOD, readings of this level cannot entirely exclude hypertension as a cause of TOD-compatible clinical signs, given minute-to-minute blood pressure variability and inaccuracies associated with blood pressure measurement. However, a suspected diagnosis of hypertension-associated damage should, of course, be carefully considered when the measured blood pressure is less than 150mmHg, and alternative explanations should be strongly considered.
Clinical signs of hypertension in the eyes can be broken down into three categories (Crispin and Mould, 2001):
In the early, often undetected, stages of feline systemic hypertension, subtle ocular findings may include focal retinal oedema and exudates, focal retinal haemorrhages, and tortuosity of the retinal vessels. Only the most attentive owners will appreciate visual deficits at this stage. In the more advanced stages, ocular findings may include retinal detachment and blindness. This is the most common point at which patients are presented for evaluation. Potential complications of systemic hypertension may include hyphaema, vitreal haemorrhage, uveitis and secondary glaucoma (Littman, 1994; Morgan, 1986; Stiles et al, 1994; Turner et al, 1990).
Clinical signs of hypertensive encephalopathy can include disorientation (which can look identical to cognitive dysfunction of older cats), depression, vestibular signs, ataxia and seizures. In humans, headaches and feelings of anxiety are frequently reported in hypertension, but determining whether these occur in cats is, of course, impossible. Many owners report subjective improvement in lethargy/depression of their hypertensive cats when antihypertensive therapy is successfully employed.
Left ventricular hypertrophy secondary to increased afterload is quite a consistent finding in hypertension (Chetboul et al, 2003), and may present as a new murmur or gallop. Congestive heart failure is a very rare consequence, but cats with myocardial hypertrophy are significantly more vulnerable to fluid overload, so congestive heart failure may develop following fluid therapy. Hypertension is a differential for any hypertrophic myocardium. It is important to remember hypertrophic cardiomyopathy does not cause hypertension.
Hypertension can occur secondary to renal disease, but is also suspected to accelerate the progression of it. Renal TOD may manifest as proteinuria or azotaemia. Successful antihypertensive therapy has been shown to reduce proteinuria (Jepson et al, 2007); however, it is not clear whether establishing normotension alters survival in these patients.
The eye has been reported to be the most vulnerable target organ for systemic hypertension in cats, with some studies suggesting a prevalence of 60% to 80% (Sansom et al, 1994; Syme et al, 2002), and some reports of hypertensive ocular lesions developing at BPs as low as 168mmHg (Carter et al, 2014; Sansom et al, 2004). While the eye is a target organ for hypertensive damage, most cases are not recognised until the disease is in an advanced stage, with the most common reason for presentation often being acute blindness secondary to retinal detachment and/or intraocular haemorrhage (Littman, 1994). The true prevalence of ocular TOD in hypertension is thus unclear, as many hypertensive cats without obvious ocular pathology do not undergo an ocular examination.
The photoreceptor layer of the retina has a very high energy and oxygen demand, relying on the underlying and highly vascular choroid to meet these needs. When detached from the choroid, the retina quickly begins the process of degeneration. Most photoreceptor death occurs within the first few days of detachment by the process of apoptosis and continues for as long as the retina remains detached (Chang et al, 1995). For the best opportunity at recovery of photoreceptor function, retinal reattachment should take place within one week (Anderson et al, 1986). Both the duration and extent of retinal detachment influence the prognosis for vision, thus early recognition of hypertensive retinopathy – prior to the development of significant retinal detachment, and, therefore, reduction/loss of vision – offers the best chance at early treatment and, therefore, prognosis (Maggio et al, 2000).
The ocular changes associated with systemic hypertension often progress over several months, so to detect subclinical ocular lesions, clinicians should aim to examine the eyes (with particular focus on the fundus) of all apparently healthy geriatric cats (more than 8 to 10 years old), as well as cats with a known diagnosis of an underlying disease process, such as chronic renal failure and hyperthyroidism, which have been linked with systemic hypertension (Carter et al, 2014; Maggio et al, 2000). Furthermore, any patient with an elevated BP, and any patient with decreased vision or complete loss of vision, should undergo a thorough ocular examination if the practitioner is to pick up early, subclinical lesions associated with systemic hypertension.
A study by Carter et al (2014) highlighted the importance of ocular examination in “at risk” patients by showing evaluation of the fundus of cats more than eight years of age allowed for the identification of patients with ocular hypertensive lesions before either the owner and/or vet were aware a problem was present. As a result, systemic hypertension could be diagnosed in the early stages, leading to earlier treatment than would normally take place and overall improved prognosis for the patient.
The blood-retina barrier is comprised of an inner and outer barrier. On the outer side, tight junctions between the retinal pigment epithelial cells regulate the passage of solutes and nutrients from the fenestrated choroidal vessels to the subretinal space. On the inner (vitreal) part of the retina, the endothelium of the retinal blood vessels (capillaries) have tight junctions and are non-fenestrated. These tight junctions aid in highly selective diffusion of molecules from the blood to the retina. The blood-retina barrier is vital to maintaining retinal homeostasis. The breakdown of the blood-retina barrier is responsible for the pathology that develops with systemic hypertension.
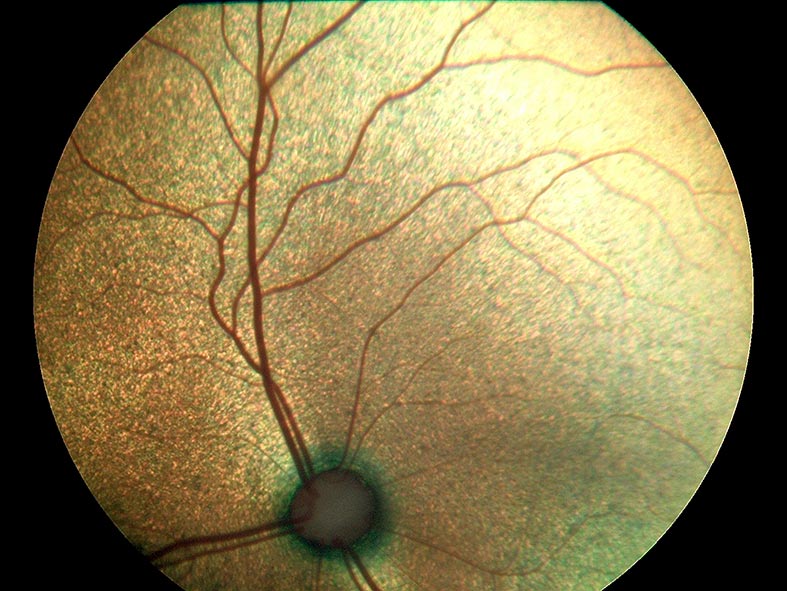
To fully appreciate the changes that develop with systemic hypertension, the practitioner must have a good understanding of the normal anatomy and appearance of the feline fundus (Figure 3). The vascular system supplying the retina and choroid differ anatomically and physiologically. Retinal arterioles exhibit autoregulation to ensure a constant blood flow, despite changes in local arterial pressure and intraocular pressure. When BP is elevated, the smooth muscle in the wall of retinal arterioles initially contracts, resulting in constriction of the arteriole and, therefore, maintenance of normal tissue perfusion. With prolonged hypertension, the clinician may observe narrowing and increased tortuosity of the retinal arterioles on examination of the fundus. When this contraction is sustained, it can result in ischaemia and retinal degeneration (Garner et al, 1975).
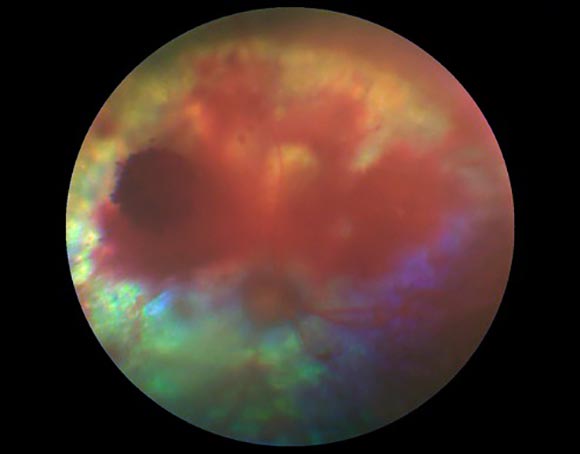
Persistent contraction may lead to the breakdown of autoregulation, thus causing dilation of the arterioles (Hayreh et al, 1986). Plasma and fibrinogen can leak into the vessel walls secondary to endothelial damage, which leads to thickening of the lumen wall and secondary occlusion. Fibrinoid necrosis occurs in the vessel wall as fibrinogen is converted to fibrin. This leads to the development of hypertensive retinopathy, as the tight junctions of the endothelial cells of the retinal vasculature break down (Crispin et al, 2001). The result is serum and red blood cell leakage into the retina, accounting for the characteristic effusive lesions (oedema, multifocal retinal oedema and intraretinal haemorrhage) seen with hypertensive retinopathy (Stiles et al, 1994; Garner et al, 1975; Figures 4 and 5). Focal retinal arteriolar dilatations (retinal arterial aneurysms) may also be detected (Carter et al, 2014; Figure 6).
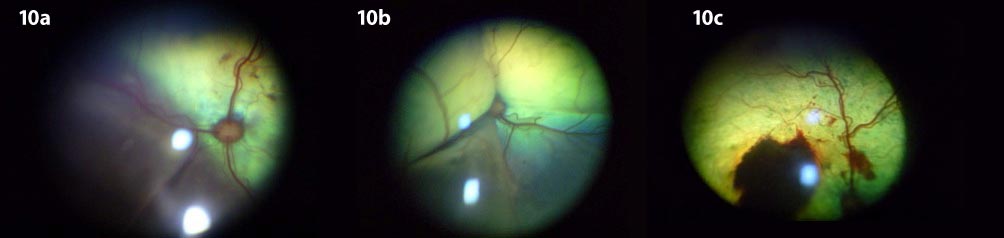
Rather than being under autoregulation, choroidal vessels are under autonomic control. Thus, during periods of hypertension, elevated circulating vasopressor substances may leak into the extra vascular space, producing vasoconstriction, ischaemia and infarction of the choriocapillaris and retinal pigment epithelium (RPE). The resultant breakdown of the RPE/epithelial blood-retinal barrier allows fluid to leak into the subretinal space, thus resulting in retinal detachment. Retinal detachment, secondary to hypertensive choroidopathy, can be variable in nature and may range from bullous to flat detachments, focal to diffuse/generalised (Hayreh et al, 1986; Figure 7 to Figure 10).
Hypertensive optic neuropathy has a more complex pathogenesis, and can result in papilloedema and optic nerve atrophy in the long term. Clinical findings of hypertensive optic neuropathy are not often appreciated in the cat, either because other changes, such as retinal oedema, haemorrhage or detachment, obscure the findings of papilloedema, or because the unmyelinated and “recessed” nature of the feline optic nerve head make the clinical features less obvious (Crispin et al, 2001).
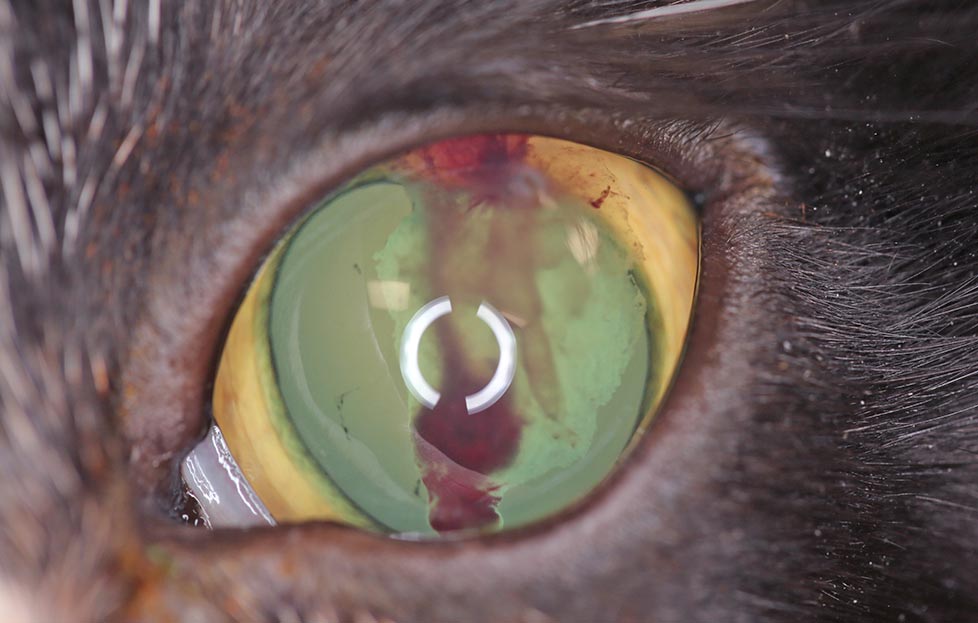
While the focal to complete bullous retinal detachment lesions seen with hypertensive choroidopathy are those characteristically observed in cats with advanced systemic hypertension, other lesions referable to high BP may also be detected, including iris aneurysms (Figure 11), hyphaema (Figure 12) and/or vitreal haemorrhages (Figure 13).
Depending on the time to detection and the severity of the intraocular changes, cats may also present in an advanced stage of secondary glaucoma. When significant hyphaema, vitreal haemorrhage, or glaucomatous changes are present, it can make complete ocular examination – in particular, fundus evaluation – more challenging and, thus, interfere with the ability to diagnose systemic hypertension. In these cases, the use of an ocular ultrasound can prove valuable for posterior segment assessment, as well as aid in ruling out obvious intraocular neoplasia as a cause for the changes detected.
Amlodipine has been shown to be an effective treatment for lowering BPs in cats with systemic hypertension and, in some cases, can lead to retinal reattachment, but not necessarily always a return to functional vision (Komaromy et al, 2004). Recovery of functional vision will be dependent on several factors, with the most important being the degree and duration of retinal detachment prior to both the diagnosis and treatment of the hypertension.
Ideally, signs of hypertensive retinopathy will be recognised early before the more advanced/severe lesions of hypertensive choroidopathy (retinal detachment), so therapy may be commenced at a time to offer the best prognosis for vision. Monitoring of ocular abnormalities should play a vital role when assessing response to therapy to achieve the best visual outcome.