22 Jul 2019
Alix McBrearty describes methods of detecting renal conditions, including blood and urine testing, as well as imaging.
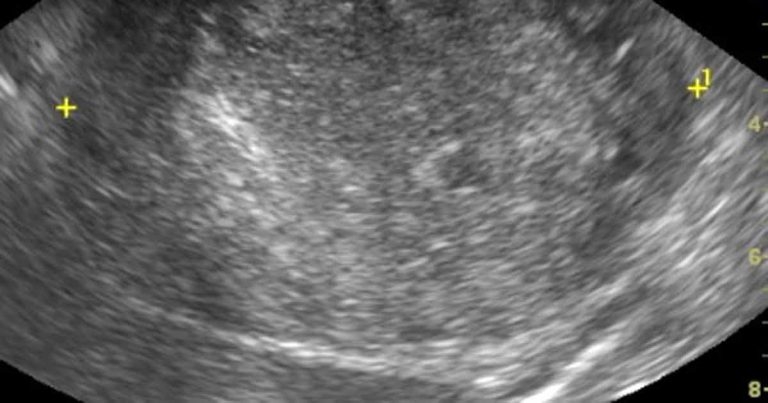
Figure 2. A long-axis ultrasound image of the right kidney of a dog. The central region of the kidney is obliterated by a rounded heterogenous, largely hyperechoic mass lesion. Histopathology of the tumour after excision showed this to be a renal carcinoma.
When we think about dogs and cats with chronic kidney disease (CKD), we often think of the patient with signs of polyuria and polydipsia (PUPD), inappetence, weight loss and vomiting; however, it is important to remember this is the end stage of a long process. Patients with CKD can have very varied presentations and, in the early stages of the disease, are likely to be asymptomatic. CKDs may first be identified because of renal dysfunction, renal inflammation or renal structural changes, and these changes may be identified incidentally. Although many of the diseases may ultimately result in the classical findings of PUPD and uraemic signs, by the time these have developed the diagnosis is relatively straightforward, and the renal changes are irreversible and extensive.
This article looks at a variety of commonly used tests that might indicate CKD and what information they provide. These will include tests on blood and urine, as well as renal and ureteric imaging. It also discusses ways we might diagnose CKD earlier – giving us an opportunity to intervene and alter the course of these diseases.
Chronic kidney disease (CKD) is any disease that results in changes to the renal structure, or a reduction in renal function, in the patient over a prolonged period (more than two or three months).
Although, at the time of diagnosis, a proportion of animals have evidence of functional kidney disease due to a reduction of glomerular filtration (causing signs of uraemia) or a loss of concentrating ability (causing polyuria and polydipsia; PUPD), these are only two of many presentations of patients with CKD. Other CKD patients present with non-specific signs, such as weight loss, abdominal pain, free fluid accumulation, abdominal masses, evidence of thromboembolic disease or hypertension.
Many patients with structural disease alone, or with early reductions in functional capacity, will be asymptomatic and may be diagnosed incidentally during investigations for other problems or on routine monitoring. CKDs are often progressive and, therefore, the identification of patients with asymptomatic early CKD is important.
It is in these patients we have the greatest opportunity for identifying treatable underlying causes, treating complications (such as proteinuria and hypertension) and of slowing disease progression, delaying the onset of more serious signs and renal-related death. The development of a staging system for CKD that emphasises the structural and functional stages of CKD by the International Renal Interest Society (IRIS; Table 1) reflects this importance and is accompanied by guidelines for management at different stages. Many of the tests covered in this article are not specific for kidney disease, so careful interpretation is needed to determine whether the problem does suggest CKD.
| Table 1. International Renal Interest Society (IRIS) guidelines for staging chronic kidney disease (CKD) | |||
|---|---|---|---|
| IRIS CKD stage | Stable fasting serum creatinine (µmol/L) | Additional notes | |
| Dogs | Cats | ||
| I | Less than 125 | Less than 140 | Non-azotaemic, but animals will have other evidence of CKD (for example, structural abnormalities, persistent poor concentrating ability with no other identified non-renal cause, renal proteinuria, increasing serum creatinine over time). |
| II | 125 to 180 | 140 to 250 | Mild renal azotaemia. Clinical signs of functional renal disease often absent. |
| III | 181 to 440 | 251 to 440 | Moderate renal azotaemia. Severity of clinical signs will vary from patient to patient. |
| IV | More than 440 | More than 440 | Severe renal azotaemia. Signs of renal functional failure and azotaemia increasingly likely. |
| Note: patients should then be sub-staged on the magnitude of their systolic blood pressure and proteinuria (visit www.iris-kidney.com for details). | |||
| Adapted from the IRIS staging of CKD (modified 2017). | |||
One of the major functions of the kidneys is the filtration of blood to excrete soluble waste as urine. Glomerular filtration rate (GFR) is considered the best indicator of overall renal function. It is the volume of ultrafiltrate produced by glomerular filtration per unit time. As GFR is not only affected by renal function, but also by renal blood flow (altered by dehydration and anaesthetics/sedatives) and flow of urine away from the kidneys, the interpretation of changes in GFR (either measured directly or indirectly) must take this into account.
Direct measurement of GFR is difficult – instead, in the clinic it is indirectly estimated by measuring urea, creatinine and/or symmetric dimethylarginine (SDMA). When reductions in renal function (GFR) are identified, it is crucial to determine whether these changes are acute or chronic (or both) as this will significantly alter patient management and prognosis. Regardless of which indirect marker is used, the large functional renal reserve limits the usefulness of these tests for the detection of mild or early kidney damage.
Urea increases as GFR falls. It is a poor marker of GFR as it is not only filtered in the glomerulus, but is also reabsorbed in the tubules at a variable rate depending on the rate of flow of urine through the nephron (for example, urea reabsorption is increased in hypovolaemia). Urea concentrations are also affected by multiple extra-renal factors, including the rate of protein catabolism, protein digestion and hepatic failure.
Serum creatinine also increases as the GFR falls, but it is less affected by extra-renal factors. It is, however, affected by breed (higher in Birman cats1) and muscle mass2 (higher in greyhounds3). Changes in muscle mass must be considered when using longitudinal sampling to monitor renal function in animals with decreasing body condition. Increased creatinine is a very insensitive marker of reduced GFR, only increasing above the reference range when 65% to 75% of renal functional mass is lost. This is particularly true when using existing reference ranges that tend to be very broad.
The IRIS staging system for CKD, which uses creatinine (and, to a lesser extent, SDMA) to classify the functional severity of CKD, suggests we should be concerned about reduced renal function in animals with creatinine values considered normal by many laboratories (Table 1), and consider further investigation and treatment at this stage. A further consideration is that the relationship between GFR and creatinine is not linear (Figure 1), which means small increases in serum creatinine at values below the reference range may indicate marked reductions in GFR. If the muscle condition of a well-hydrated patient has not changed and creatinine concentrations are increasing over time, it is highly likely the patient’s GFR is falling and it might benefit from management of its CKD.
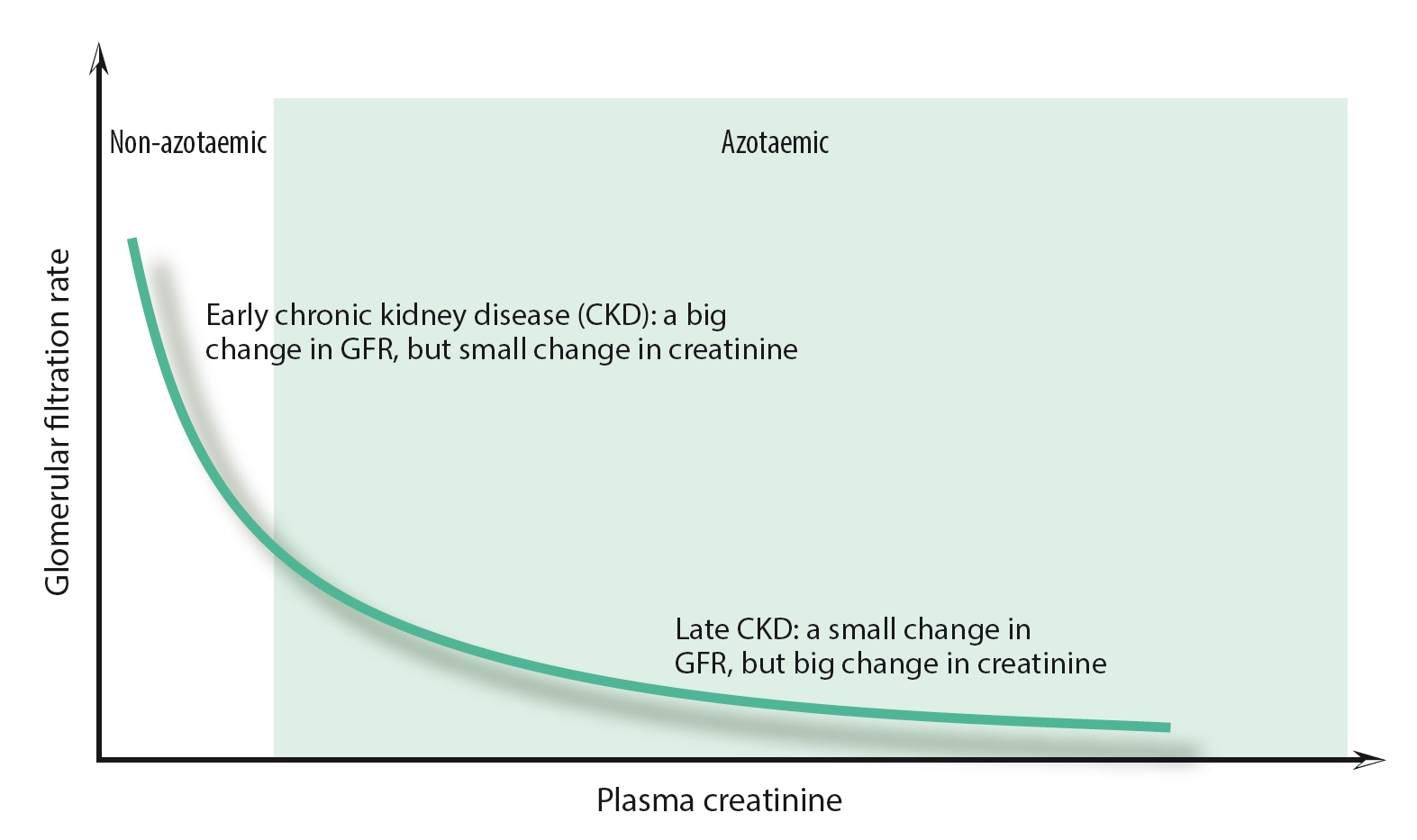
In summary, creatinine can be used to detect renal disease earlier if we start to react to values within the upper reference range and to increasing values in individual patients over time. Opportunities to do this may arise in dogs and cats routinely attending clinics for wellness checks.
SDMA is cleared mainly by glomerular filtration and is another indirect measure of GFR, with levels increasing as GFR declines. SDMA concentrations are higher in healthy greyhounds5, and in puppies and kittens younger than one year old. In several studies of dogs and cats with progressive CKD, SDMA has been found to increase above the reference range earlier than creatinine6-8, although a study in dogs using a creatinine cut-off of 115µmol/L (well within most normal reference ranges) suggested the sensitivity and specificity of creatinine and SDMA for the detection of reduced GFR were similar9.
Persistent increases in SDMA, despite a normal creatinine, likely suggests early CKD. Unlike creatinine, SDMA is not affected by lean body mass2,10 and, therefore, has a useful role in monitoring declining GFR in patients with poor or decreasing muscle mass. SDMA is of little additional benefit in patients with high creatinine concentrations and normal stable muscle condition.
Proteinuria may be identified using a urine dipstick. Excessive urine protein may be identified due to: excess systemic protein production with overflow into the urine (pre-renal proteinuria); failure of the glomerular filtration barrier to prevent protein filtration or failure of uptake of normally filtered protein (renal proteinuria); or urinary tract inflammation (post-renal proteinuria). Biochemistry profiles and urine microscopy should be performed to rule out pre-renal and post-renal causes before any conclusion proteinuria is due to renal disease. False positive and negative protein urine dipstick results, and transient proteinuria, can also occur, and all should be excluded prior to lengthy investigations. Proteinuria, thought to be due to primary renal disease, should be quantified by calculating the urine protein:creatinine (UPC) ratio.
Results greater than 2 suggest glomerular damage, whereas values less than this may be due to glomerular or tubular damage. Identification of renal proteinuria should prompt further investigation as it can lead to progressive renal damage, low serum albumin, predisposition to thromboembolic disease and systemic hypertension.
Casts are formed within the renal tubules and a small number of hyaline casts or granular casts may be seen on urine microscopy in normal animals; however, large numbers of hyaline casts may be identified in patients with significant proteinuria and, if identified, should prompt further investigation.
Patients with suspected glomerular disease may benefit from renal biopsy to further characterise their disease; however, this should not be undertaken without a thorough understanding of sample collection and processing, and the importance of biopsy examination by dedicated nephropathologists.
Another major function of the kidneys is to maintain water balance within the body by retaining or eliminating water as appropriate. For this to occur, there must be enough nephrons and those nephrons must be able to respond appropriately to antidiuretic hormone (vasopressin) to concentrate urine. Urine specific gravity (SG) is used as a measure of urine concentration. It is easily measured using a refractometer – ideally, testing the patient’s first urination in the morning, which should be maximally concentrated.
In healthy dogs and cats, urine SG can vary from 1.001 to greater than 1.080 – depending on water intake. Large variations are seen in healthy animals. Urine SG may be affected by non-renal conditions, such as diabetes mellitus, central diabetes insipidus and hepatic failure, as well as the administration of drugs (for example, diuretics or glucocorticoids).
In animals with progressive tubular dysfunction or nephron loss, urine-concentrating ability will be progressively reduced. Urine SG persistently less than 1.035 in cats and less than 1.030 in dogs, in the absence of other disease or drug administration, is suggestive of reduced concentrating ability and tubular dysfunction/nephron loss. It is this reduction in concentrating ability that results in the clinical signs of PUPD. A single result less than these values is also inappropriate in a clinically dehydrated patient. Reduced urine concentrating ability is an insensitive test of reduced renal function as it is not observed until a reduction of approximately two-thirds has occurred and cats may retain renal concentrating ability despite even greater renal functional loss.
The presence of glucose on urine dipstick testing in a normoglycaemic animal is suggestive of renal tubular damage. Glucose is freely filtered in the glomerulus and then almost entirely reabsorbed from the glomerular ultrafiltrate in the proximal tubules. If the proximal renal tubules are dysfunctional, failure to remove glucose from the ultrafiltrate can occur and result in glucosuria.
Proteinuria may also be identified with tubular damage as the tubular cells may fail to remove the small amounts of protein normally present in the glomerular ultrafiltrate. The magnitude of tubular proteinuria is expected to be low (UPC less than 2).
The presence of cellular or waxy casts, or large numbers of hyaline or granular casts on urine microscopy, suggest active pathology within the renal tubules. Their absence does not, however, rule out kidney disease or tubular injury. The presence of large numbers of cuboidal to low columnar epithelial cells in the urine is also suggestive of an active tubular lesion; however, the presence of low numbers is normal. It must be determined whether chronic underlying disease is also present in these cases.
Glucosuria, proteinuria and the urine microscopy changes described are insensitive tests of renal tubular dysfunction and their absence does not rule out renal tubular damage.
Positive results on urine dipstick analysis for blood or identification of red blood cells on urine microscopy may suggest renal haemorrhage. This can occur in cases with nephroliths, pyelonephritis and glomerular diseases, which may have few obvious clinical signs. Positive dipstick reactions can also occur in the presence of haemoglobin or myoglobin. Centrifugation or microscopy will help determine whether intact erythrocytes or haemoglobin are present. An absence of lower urinary tract signs (for example, stranguria, pollakiuria) in cases with haematuria should prompt consideration of renal disease as the cause.
The identification of white blood cells (WBC) on urine microscopy may suggest the presence of pyelonephritis or inflammation of the lower urinary tract, or both. Pyelonephritis can be difficult to identify and is likely under-diagnosed. Whenever WBC are identified in urine, the possibility of pyelonephritis should be considered.
Further investigations to determine renal involvement might include evaluation of creatinine, SDMA and renal ultrasound. It is important to remember leukocyte and nitrate dipstick reactions are not valid or useful for the identification of urinary tract inflammation in dogs and cats.
Additionally, it must be noted patients with some conditions, such as diabetes mellitus and hyperadrenocorticism, may not have WBC in the urine, despite a positive urine culture.
*The cortex is normally hyperechoic to the medulla. An increase in the echogenicity of the cortex and medulla result in loss of corticomedullary definition. In small dogs, increased medullary echogenicity may be normal, but in larger dogs and cats, this finding suggests chronic kidney disease.13
Renal ultrasound is non-invasive, not painful and useful to identify renal structural abnormalities. Ultrasound does not provide information about renal function. CKDs that can result in structural changes include renal tumours (including lymphoma and adenocarcinoma; Figure 2), renal dysplasia, pyelonephritis, nephrolithiasis and polycystic kidney disease. Subtle structural changes are seen with conditions such as chronic interstitial nephritis and glomerular diseases until later in the disease process when the corticomedullary definition is reduced, and the kidneys become small, with irregular contours.
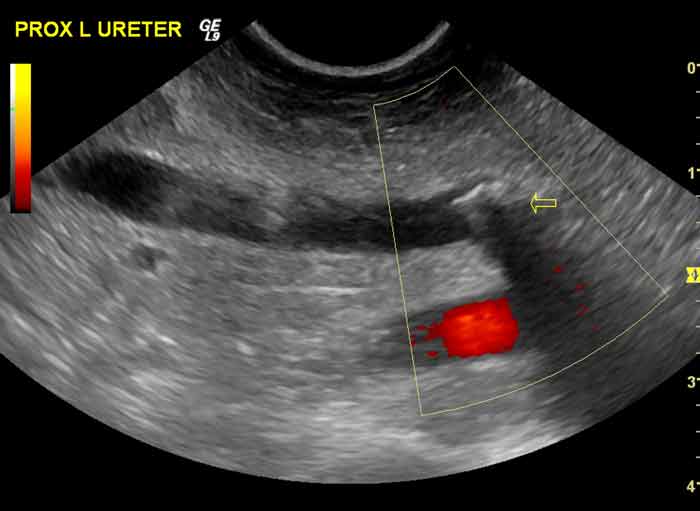
Ultrasound can be used to identify ureteroliths (Figure 3) and the resulting degree of ureteral and renal pelvic distension. It can also be used to direct fine needle aspirates in cases suspected of having tumours or for collecting renal cortical biopsies. Doppler ultrasound can be used to qualitatively or quantitatively assess renal blood flow in the kidney11.
Survey abdominal radiography gives information about the size, shape, margination and opacity of the kidneys (Figure 4); however, ultrasound is often prioritised in cases with suspected CKD as it gives more information about the renal architecture. Radiography remains very useful for the identification, characterisation, quantification, and to determine the position of nephroliths and ureteroliths, which are thought to be becoming more common – particularly in cats12. The views of choice are right-lateral (improves visualisation of both kidneys) and ventrodorsal.
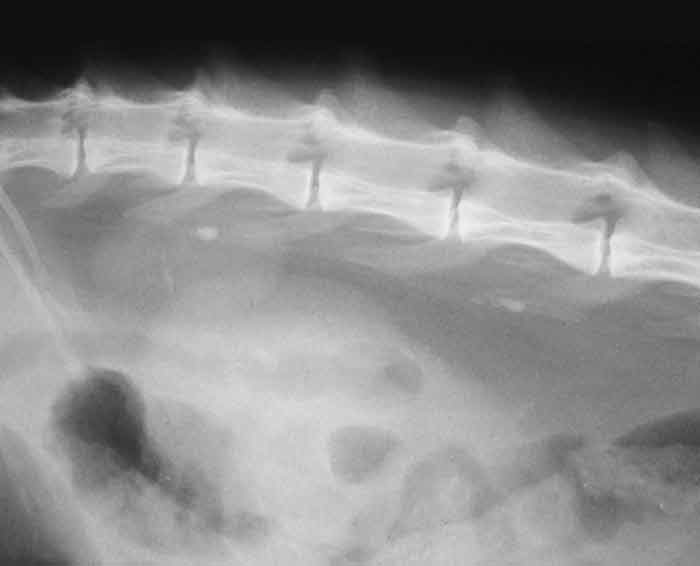
Excretory urography may be used to help determine whether a kidney has any qualitative function, and the degree of ureteral obstruction by ureteroliths, as well as allowing clearer delineation of the renal parenchyma and pelvis, but careful prior consideration must be made of the risks associated with contrast administration – particularly in patients with azotaemia.
The identification of kidney disease using these tests is just the first step in the management of these patients. We must then decide whether the disease is acute or chronic (or both), attempt to make a definitive diagnosis of the underlying disease, identify underlying causes and complications, and decide how best to manage the patient.
As increasing numbers of practices perform routine wellness checks on asymptomatic animals, the number of animals diagnosed with kidney disease at an early stage should rise and, alongside improvements in patient management, should improve outcomes in this group of patients.