26 Mar 2018
Gerard Olivares and Mellora Sharman discuss the underlying aetiology of this issue – including causes, advances in treatment and the use of probiotics. Includes video content.
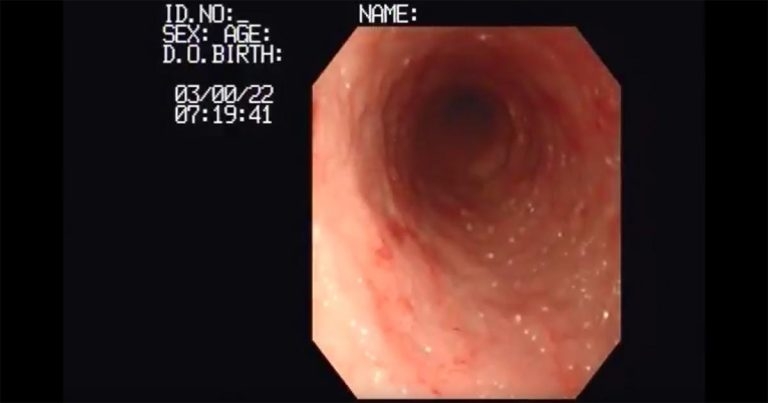
A screenshot from Video 1, which shows an endoscopic view in the duodenum of a dog with lymphangiectasia.
Inflammatory bowel disease (IBD) is used as a collective term to describe disorders of the gastrointestinal (GI) tract characterised by persistent or recurrent GI signs – such as vomiting, diarrhoea, weight loss, borborygmus and hyporexia – as well as histological evidence of intestinal inflammation and exclusion of identifiable underlying causes for the inflammation.
The term IBD has been adapted from human medicine, where disorders include Crohn’s disease and ulcerative colitis. The term chronic enteropathy (CE) has been suggested as more appropriate for veterinary use, as it recognises the difference between these diseases in most of our patients compared to human medicine1.
CEs have historically been defined by their histological appearance. This provides little information as to their aetiology; indeed, many cases have no obvious histological change. More recently, CEs have been defined by their response to empirical treatments trialled sequentially – such as food-responsive enteropathy (FRE), antibiotic-responsive enteropathy (ARE), immunosuppressant-responsive enteropathy (IRE) and non-responsive enteropathy (NRE)2. Therefore, the term IBD in canine patients is best used for cases that have failed to resolve with diet and antibiotics, but have responded to immunosuppressive medication.
The aetiopathogenesis of IBD in cats is believed to be similar to dogs, but differences exist in the course of the disease and management that will be discussed in this article.
The aetiology of CE is not completely understood, but it is believed inflammation develops in a genetically predisposed animal, triggered by the breakdown of immunological tolerance to luminal antigens, such as dietary components and the intestinal microbiota3.
Mutations in innate immune receptors of humans (nucleotide-binding oligomerisation domain-containing protein 2) and dogs (toll-like receptor [TLR] 4 and TLR 5) have been linked to CE susceptibility. In the presence of an enteric microbiota, it may lead to up-regulated proinflammatory cytokine production – for example, interleukin 17 and tumour necrosis factor alpha – and reduced bacterial clearance, thereby promoting chronic intestinal inflammation4.
The roles of the microbiota and dietary antigens have been widely studied5,6. The predisposition of certain dog breeds – for example, boxers and German shepherd dogs – and their clinical response to antibiotics point to an interaction between host genetic susceptibility and intestinal microflora7. Studies have shown microbial communities found in dogs with CE are significantly different to those in healthy dogs – strengthening the idea of a correlation between microflora and CE8,9.
In dogs, the interaction of genetics and diet is supported by the finding gluten-sensitive enteropathy in Irish setters is an autosomal recessive trait, while adverse reactions to corn, tofu, cottage cheese, milk, lamb and farina cream of wheat have been described in soft-coated wheaten terriers6,10. Whether similar pathologic mechanisms come into play in the development of idiopathic feline IBD has not been fully determined11.
These studies suggest chronic intestinal inflammation in CE may be due to overly aggressive T cell responses to enteric bacteria, or fungi, in hosts with genetic defects that regulate bacterial killing, mucosal barrier function or immune responses. Environmental factors likely govern inflammation onset, or reactivation, and modulate genetic susceptibility to disease11. Irrespective of the specific causative agent, the end result is GI inflammation and observed clinical signs.
A diagnosis of CE usually involves careful evaluation of signalment, home environment, history, physical examination, clinicopathological findings, diagnostic imaging and histopathology of intestinal biopsies7. CE is diagnosed in patients with chronic GI signs (longer than three weeks). Most often, these cases are presented with vomiting, diarrhoea and weight loss.
The initial approach to GI signs is based on determining its nature, severity and localisation. Although diffuse GI disease is most common in CE cases, evaluation of clinical signs may help refine the region of most interest and allow identification of “alarm” signs that may warrant more aggressive diagnostic investigation and treatment. Identification of haematemesis, inappetence or weight loss are more likely to be associated with gastric or upper small intestinal disease, while haematochezia, mucoid faeces or tenesmus would be more commonly expected with large intestinal disease7.
The diagnostic approach to patients with chronic GI signs is summarised in Panel 1. Careful evaluation of signalment, history and physical exam can identify atypical clinical signs that can prompt consideration of specific primary GI or extra-GI disorders. Although initial stages of investigation may depend on the presenting clinical signs, they are most likely to include evaluation for systemic, metabolic or infectious causes via haematology, biochemistry, urinalysis, and faecal analysis for parasites and other enteric pathogens.
1. Integrate signalment, history and physical exam
History:
Thorough physical examination:
2. Faecal examination
Consider analysis for egg count, culture and ELISA/PCR (for example, Giardia, Cryptosporidium, Salmonella and Campylobacter species)
3. Clinicopathological testing
Evaluation for non-GI disease:
Assess for evidence of primary GI or extra-GI disease, or risk factors for severe disease. Common findings include:
Additional tests to perform or consider:
4. Diagnostic imaging (radiographs and abdominal ultrasound)
Exclude non-GI disease:
Evaluate and characterise GI disease:
Serum concentrations of folate and cobalamin should be measured, as they may identify the need for supplementation and guide disease localisation – folate is absorbed in the duodenum, while cobalamin is absorbed in the ileum.
Where suspected on the basis of signalment or clinicopathological findings, additional testing for specific conditions – such as hypocortisolaemia, chronic pancreatitis or exocrine pancreatic insufficiency – may be warranted. For example, a haematology profile lacking evidence of an inflammatory or stress leukogram in a clinically unwell patient may prompt testing for hypocortisolaemia via measurement of baseline cortisol, or by performing an adrenocorticotropic hormone stimulation test.
Dogs with a history of intermittent vomiting and/or cranial abdominal discomfort, or a historic suspicion of intermittent abdominal pain, may lead clinicians to test for chronic pancreatitis with a quantitative pancreatic lipase test, although chronic pancreatitis may also occur concurrently with CE in dogs.
This is, perhaps, a particularly important consideration for cats, as concurrent inflammatory disease involving the liver or pancreas (feline triaditis) can be present and may contribute to clinicopathological findings11.
Exocrine pancreatic insufficiency, both in dogs and cats, is an important differential for CE and can be reliably excluded in most cases through measurement of trypsin-like immunoreactivity. It may also occur secondary to chronic pancreatitis, which may occur in association with – or as a result of – CE.
Survey radiography may allow exclusion of surgical disease by detection of foreign bodies, free peritoneal gas, intestinal displacement, masses, decreased serosal detail or suspicion of obstructive ileus.
Ultrasonography has greater sensitivity than radiography and excellent specificity for the detection of lesions – such as intussusceptions, intramural masses, and radiopaque and radiolucent foreign bodies – along with changes to the intestinal wall such as thickening, loss of layering, and mucosal striations and speckles consistent with lymphangiectasia (Figure 1).
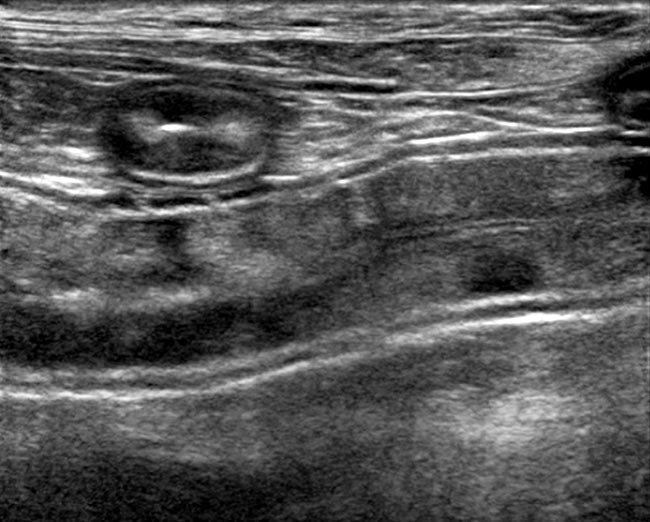
Lymphadenopathy may also be more easily identified using ultrasound in both chronic inflammatory or neoplastic enteropathies. Furthermore, ultrasound may allow guided fine needle aspiration of abdominal structures – such as lymph nodes or thickened intestinal walls – for cytologic examination, which may be useful for obtaining a definitive diagnosis in certain disease situations, such as some neoplasia.
Although abdominal ultrasound has historically been a routine part of the investigation of the GI system, diagnostic utility in cases of chronic vomiting or diarrhoea has been questioned and, more often than not, ultrasound is normal12,13.
Once exclusion of underlying disease has been completed, chronic GI signs may be explained by FRE, ARE, idiopathic IBD (IRE or NRE) or primary lymphangiectasia7.
Whether treatment trials or more invasive investigation – such as endoscopic or surgical biopsy – is performed at this stage may depend on owner factors, the clinicopathological findings and the clinical stage or severity of disease. Clinical staging has been well established in dogs (canine IBD activity index and canine CE clinical activity index) and cats (feline CE activity index).
Similar to other indices, the magnitude of the numerical score is proportional to the degree of inflammatory activity. This can be helpful in the diagnostic approach and management of patients, both as a measure of initial response to treatment and to assess long-term progress14-16.
In cases with high severity of clinical scores – along with alarm signs such as haematemesis, melena (Figure 2), severe weight loss, ascites (Figure 3), or the presence of panhypoproteinaemia, hypocobalaminaemia, marked intestinal thickening or mesenteric lymphadenopathy – the recommendation is to perform an intestinal biopsy to define the cause, exclude more sinister disease and, potentially, optimise therapy7.
While biopsy of the mucosa for histopathological diagnosis of IBD has historically been considered obligatory, the benefit of endoscopy and biopsy for cases where dietary trials have failed – but underlying, more sinister or infectious disease is unlikely and immunosuppressive therapy is most likely to be the next step – has been discussed. In this situation, geographic location, clinicopathological index of suspicion for more sinister or infectious disease, and owner factors – including financial – may impact whether further, more invasive diagnostic testing is performed.
Where biopsy is performed, samples may be obtained via endoscopy (mucosal sample), laparoscopy or exploratory laparotomy (full-thickness sample). Endoscopy allows visualisation of the intestinal mucosa (Figure 4), which may raise suspicion of a specific process (for example, lymphangiectasia; Video 1).
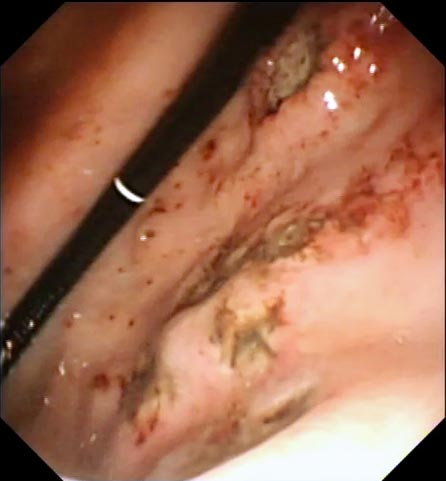
Endoscopy also allows collection of multiple tissue samples without the need for invasive surgery. This minimises recovery times and allows earlier intervention with some therapies (Video 2).
A combination of gastroduodenoscopy and colonoscopy allows examination and sampling of most of the intestinal tract – except the mid-jejunum – where the ileum is able to be intubated during colonoscopy (Figure 5). Evaluation of both the upper and lower GI tract – and, particularly, inclusion of the ileum – is of clinical importance, given histological disease can be more readily observed in ileal biopsies compared to duodenal biopsies17.
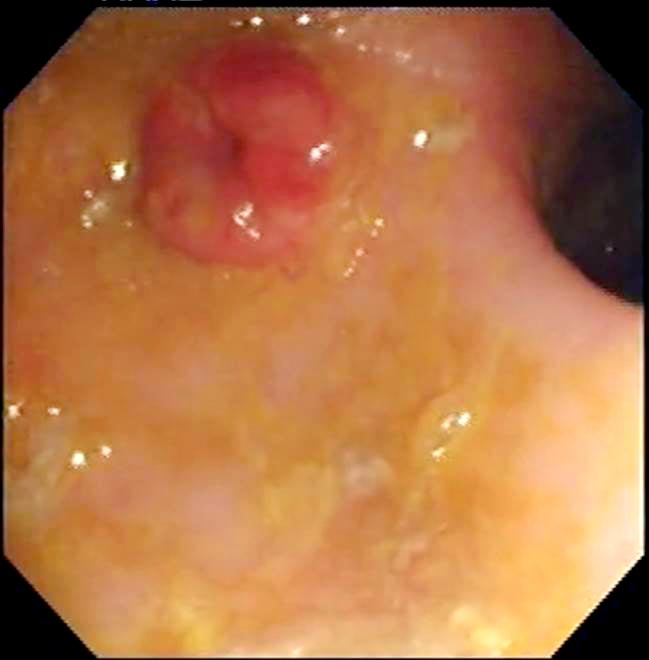
Endoscopy alone should never be performed for investigation of GI disease, as gross evaluation of the mucosa may not match the microscopic picture and mucosal biopsy is always warranted. WSAVA guidelines suggest a minimum of six endoscopic biopsies are collected from each region (gastric, duodenal, ileal and colonic)18.
Most often, endoscopic biopsy is sufficient for diagnostic needs. Surgical biopsy may be a consideration when endoscopic equipment is not available, disease is evaluated to be less accessible for endoscopic biopsy or focal intestinal lesions, or when previous endoscopy biopsy findings do not adequately explain the clinical picture.
Controversy exists in feline medicine concerning the relative diagnostic accuracy of endoscopic versus full-thickness specimens for the diagnosis of IBD and alimentary lymphoma11. Traditionally, full-thickness biopsy has been felt to be superior for differentiation between small cell lymphoma and IBD in cats, although development and evaluation of new strategies – such as immunohistochemistry and PCR for antigen receptor rearrangement – may help overcome this19-21.
Cats with a suspicion of multiple organ diseases, such as triaditis, may also be better managed by coeliotomy or laparoscopy, allowing biopsies of the liver and/or pancreas to be obtained with full-thickness intestinal biopsies11. Regardless, the risk of dehiscence after surgical biopsy can be substantial – especially if the patient is malnourished, hypoproteinaemic or the tissue is neoplastic; therefore, careful consideration and discussion is warranted before an approach is agreed.
Standard histological techniques have not been shown to aid in differentiating between FRE from ARE, IRE or NRE, but this step is crucial in excluding other diseases with a similar clinical presentation to IBD – for example, GI lymphoma and histiocytic ulcerative colitis15.
Histopathological scoring schemes and standardised criteria have been suggested by the WSAVA18. Typical microscopic findings in CE consist of mild to severe inflammatory cell infiltration of the GI mucosa, accompanied by varying degrees of mucosal architectural disruption. Lymphocytic-plasmacytic enteritis is the most common histological manifestation of intestinal inflammation, although this inflammatory type is non-specific for differentiating between underlying causes.
The presence of an eosinophilic infiltrate is the second most common form of idiopathic IBD. Eosinophilic mucosal infiltrate has also been associated with dietary sensitivity, presence of endoparasites, hypocortisolaemia, and mast cell tumours22. Intestinal infiltration with macrophages or neutrophils raises the possibility of an infectious process, and culture, special stains and/or fluorescence in situ hybridisation are indicated.
Newer technologies have been described for investigating chronic GI signs, primarily in dogs. These include capsular endoscopy and double-balloon endoscopy, which may help overcome the limitations of access to some regions of the GI tract – such as the jejunum – and/or the need for clinical investment in expensive equipment. Novel technologies – such as confocal endomicroscopy – may allow real time, biopsy-equivalent assessment, and provide the means for assessment of the mucosal function and permeability, including provocative testing to determine the underlying aetiology of disease23.
The therapeutic approach to dogs and cats with CE also depends on the severity of the disease, as well as clinicopathological and histological findings. As aforementioned, application of clinical scoring systems may help evaluate clinical severity and prioritise cases that would benefit from more aggressive diagnostic and therapeutic intervention, as well as allow prognostication.
Stable patients with chronic GI clinical signs and normal albumin concentrations can be given the option of empirical treatment trials, initially with diet. Dietary strategies may include use of hypoallergenic or novel protein diets, low-fat and easily digestible diets, or alteration of the fibre content.
A dietary trial with hydrolysed diet or antigen-restricted/novel protein source should be performed for a minimum of one to two weeks, depending on the frequency of clinical signs. Longer treatment trials may be needed for cases where less frequent, intermittent clinical signs are reported. Positive response to dietary trials alone in dogs with lymphoplasmacytic enteritis has been between 60% and 88%7. In one study, dietary therapy alone was successful in half of cats with IBD24.
A single dietary approach is unlikely to suit all clinical cases, and the authors advise clients numerous dietary strategies may need to be tried before failure to respond to diet is concluded. A true dietary trial should always include a challenge phase, where relapse of disease is confirmed on introduction of the previous diet; however, many owners are reluctant to perform this step.
Antibiotic treatment trials with metronidazole or oxytetracycline have been used in conjunction with dietary change and/or immunosuppression for cases failing dietary management, or that have specific infectious agents identified, and before immunosuppressive therapy is recommended. Common choices for antibiotics in dogs include oral administration of tylosin (10mg/kg to 15mg/kg every 8 hours), oxytetracycline (10mg/kg to 20mg/kg every 8 hours) or metronidazole (10mg/kg every 12 hours) for 14 to 28 days7. In cats, an antibiotic trial with metronidazole (15mg/kg orally every 24 hours) for 14 days is advised11.
These therapies can be tapered to the lowest effective dose, then ceased, or continued at the lowest dose long term. If signs recur after stopping treatment, long-term, low-dose antibiotic therapy can be used, while intermittent, short-term (one to two weeks) therapy during disease flares has been previously recommended7,11. Response to these antibiotics may occur due to modification of the GI microbiota and/or immunomodulatory effects.
The use of antibiotic therapy in such a manner is controversial, given an age of increasing antibiotic resistance. Increasingly, a move is occurring towards reserving antibiotic treatment trials for patients that fail to respond to dietary and immunosuppressive treatment trials, rather than recommended initially or as part of a “global” treatment protocol. In one study, prednisolone was as effective as prednisolone-metronidazole for induction therapy of canine CE25. Antibiotics may, therefore, not provide additional benefit for induction of remission, but contribute to resistance within the bacterial population.
Broad-spectrum antibiotics, such as cephalosporins or amoxicillin-clavulanate, are rarely warranted for primary management of chronic GI signs.
Where not already performed, failure to respond to empirical therapy – or worsening of disease – is an indication for endoscopy and intestinal biopsy7. As aforementioned, endoscopy and biopsies are also recommended in preference to treatment trials where severe clinical disease exists based on clinical scoring schemes, or where patients have identified protein-losing enteropathy (PLE). This aids exclusion of more sinister disease, or specific infectious agents depending on geographic location, before immunosuppression is started.
A number of options exist for immunosuppressive protocols in dogs, and each have their benefits and potential adverse effects. Glucocorticoids, usually prednisolone, are typically used in most cases as a first-line treatment. Dosing protocols can vary, but are often based on a 2mg/kg/day or 30mg/m2 oral protocol over an initial two-week period, before considering treatment alterations or dose tapering. On remission, the dose can be gradually tapered every three to four weeks by approximately 25% to 50%26.
A similar starting dose of prednisolone has been recommended in cats for the first two weeks, with a tapering dose of 25% at two to four weekly intervals as directed by clinical response11.
Budesonide – a glucocorticoid with extensive first pass metabolism and, therefore, reduced systemic side effects – was shown to have similar remission rates (more than 65%) compared with prednisolone when given over a six-week period27.
Alternatively, other adjunctive immunosuppressant medications can be used in addition to glucocorticoids, potentially allowing more rapid dose reduction, limitation of steroid side effects and avoidance of the development of prednisolone refractory disease. Limited data is available on response to the most efficacious adjunctive immunosuppressive therapy in dogs. Options include azathioprine (2mg/kg or 50mg/m2 daily for two weeks then every other day [EOD]), ciclosporin (5mg/kg/day to 10mg/kg/day split dosing) or chlorambucil (4mg/m2/day to 6mg/m2/day then EOD).
In dogs, azathioprine is historically the most commonly recommended adjunctive therapy and many immunosuppressive treatment protocols are based around the use of this drug7. While efficacious in this disease, azathioprine can have variable onset in its action – making responses difficult to predict. Hepatotoxicity and myelosuppression are potential side effects, and regular monitoring with periodic repeated blood profiles is recommended. This can add to costs, making it as expensive as alternatives. In cats, azathioprine in particular is not a routinely used immunosuppressant because the low activity of thiopurine methyltransferases can increase the risk of adverse effects, including death.
Ciclosporin provided improvement in 12 of 14 dogs with steroid-refractory CE, with a reduction in clinical activity index and reduction in T cell numbers in duodenal biopsies following therapy28. Markedly variable blood concentrations can be seen among patients receiving ciclosporin and therapeutic drug monitoring may be achieved via monitoring trough or peak ciclosporin drug concentrations; however, adjusting levels on the basis of these concentrations remains challenging. Pharmacodynamic assessment, via evaluation of interleukin 2 expression, may be more accurate29, but can be more difficult to access as a test.
In cats, the use of chlorambucil is usually recommended in refractory cases, or where small cell lymphoma and IBD cannot be easily differentiated. This medication is usually well tolerated by most cats and is minimally myelosuppressive. It is ideally dosed at 2mg/cat every 2 to 3 days, although an alternative dosing regimen of 20mg/m2 every 14 days has also been used11.
One small, retrospective study has assessed the use of chlorambucil in dogs with IBD and PLE30. While this study suggested chlorambucil-prednisolone therapy was more efficacious for treating PLE compared with azathioprine-prednisolone, the design and nature of the study was poor and results may be due to random chance alone.
Given limited evidence to support each option, choice of adjunctive medication is often largely a matter of personal preference as published comparative studies are lacking2. With positive response to therapy, these medications can also be tapered over a period of months.
As aforementioned, cobalamin supplementation is a vital component of treatment of CE if hypocobalaminaemia is identified, as cobalamin is important for cellular function and can reduce the effectiveness of other treatments. Treatment is generally recommended when cobalamin concentrations are lower than 270ng/L, as evidence of cellular dysfunction via measurement of methylmalonic acid concentrations has been demonstrated with both low normal and low concentrations of cobalamin31.
Supplementation has traditionally been with parenteral therapy; however, evidence suggests oral supplementation is effective in many cases. A reasonable guide to parenteral or oral cobalamin supplementation can be found on the Texas A&M University website32.
Hypercoagulability in dogs with PLE has been reported, but the mechanisms responsible are likely to be complex, and not just due to loss of proteins and, in particular, antithrombin alone33,34. Due to the potential risk of thromboembolic disease in PLE cases, the use of antithrombotics – for example, ultra-low dose aspirin (0.5mg/kg/24h)7 or clopidogrel (2mg/kg to 4mg/kg every 24 hours) – can also be considered, but further studies are needed to assess risk and benefits of therapy in these patients.
Additional short-term supportive treatments may also be useful in some cases – including antacids (omeprazole, ranitidine), antiemetics and prokinetics (maropitant, metoclopramide), and feeding tube placement. Some clinicians choose gastroprotectants routinely during administration of glucocorticoids; however, no evidence exists to suggest they reduce the risk of adverse effects.
Concerns about long-term antibiotic use have prompted investigation of other methods of achieving the same results. The two main treatments with this aim are the use of probiotics, prebiotics and synbiotics, and faecal transplantation.
Since probiotics, or similar, are potentially capable of altering the microbiota and immune responses in the gut, this may be a promising future tool for CE risk reduction and management. Limited studies have evaluated a probiotic effect in healthy dogs or those with CE35-37.
A study of 20 dogs with CE, treated for 60 days with either a combination therapy (prednisone and metronidazole) or probiotic containing a mixture of strains belonging to different bacterial species, found a protective effect of the probiotic decreased clinical and histological scores, and decreased cluster of differentiation 3+ T-cell infiltration in dogs with IBD. The probiotic-treated group also demonstrated significantly enhanced regulatory T cell markers and normalisation of gut dysbiosis38. The study showed the probiotic may be successful in IBD therapy in dogs, and called for further research in the area of probiotics and IBD.
Faecal transplantation is an interesting option to treat acute and chronic diarrhoea in dogs; however, much more needs to be understood regarding its best performance, safety and usefulness before it can be recommended as a routine treatment39.
Various clinical signs may be associated with chronic enteropathies in dogs, and a sensible approach is required to differentiate and investigate primary GI versus extra-GI disease. The aggressiveness and extent of diagnostic investigation versus treatment trials will be determined by a number of factors, including the clinical presentation and the severity of disease.
A variety of treatment options, including novel therapies, are available. The approach may depend on individual cases and clinician preference in the face of limited comparative studies.
Novel approaches – such as prebiotics, probiotics or synbiotics, or faecal transplants – may provide options for cases refractory to more traditional treatments.