20 Jun 2016
Jordi Lopez-Alvarez recalls the case of a seven-week-old cocker spaniel puppy that presented with a fast heart rate, before discussing how a patent ductus arteriosus develops.

Figure 6. M-mode obtained from the right parasternal short axis view at the level of the papillary muscles showing and hypodynamic left ventricle. Fractional shortening 20% (reference greater than 25%).
Patent ductus arteriosus is probably the third most common congenital cardiac abnormality in veterinary cardiology, just after subaortic stenosis and pulmonic stenosis. The severity of disease depends on the amount of shunted blood from the systemic to the pulmonic circulation (left-to-right shunt), potentially leading to congestive heart failure.
Treatment of congestive heart failure is directed at alleviating hypoxia, including oxygen and forced diuresis. Severe congestive heart failure signs may require mechanical ventilation to treat both hypoxaemia refractory to conventional therapy and ventilatory failure (respiratory muscle fatigue).
A seven-week-old male working cocker spaniel was referred for investigation and treatment of a suspected patent ductus arteriosus (PDA).
The owners presented to the referring vet because they felt the puppy’s heart rate was faster than the other puppies in the litter and they could feel it “bouncing” in the chest. Also, despite being similar in size at birth, the puppy was failing to grow as fast as its siblings and was visibly smaller than the rest.
The owners reported a rapid deterioration in the puppy’s mentation over the previous 24 hours, from being very active and alert to progressively quieter and dull. On the car journey from the referring vet’s practice to the hospital, the owners had become very concerned as they noticed the puppy was progressively breathing faster and was very depressed.
On admission, the puppy was unresponsive, presenting both tachypnoea (more than 80 breaths per minute) and tachycardia (more than 200 beats per minute), with a regular heart rhythm.
Its bodyweight was 2.4kg and its body condition score was two out of five. Its mucous membranes were pale grey with prolonged capillary refill time and its peripheral pulses were hypokinetic and barely detectable. Auscultation of the chest revealed intense crackles throughout the lung fields and a harsh, continuous, left-sided, machinery murmur with palpable precordial thrill.
Given the history and physical examination findings, the most likely diagnosis was congestive heart failure caused by a congenital cardiac abnormality. An IV cephalic catheter was placed, while a brief Doppler echocardiographic evaluation confirmed the presence of a large PDA vessel (Figure 1) with continuous flow from the descending aorta to the pulmonary artery (Figure 2).
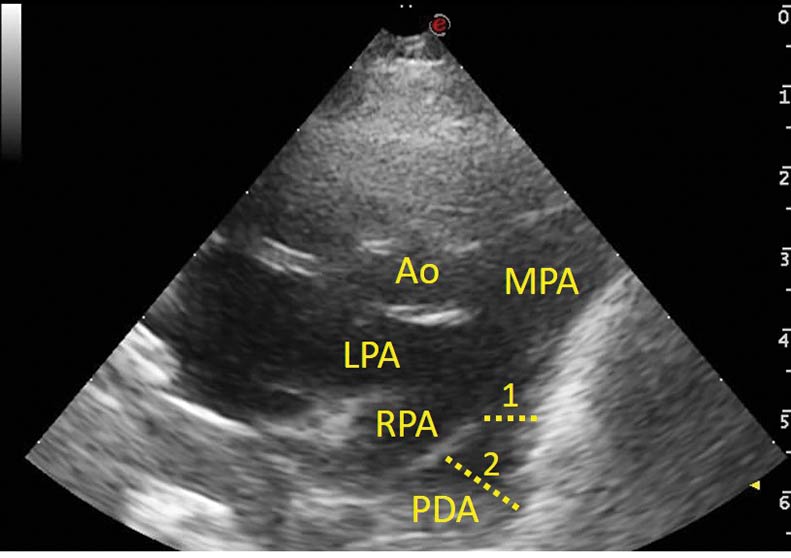
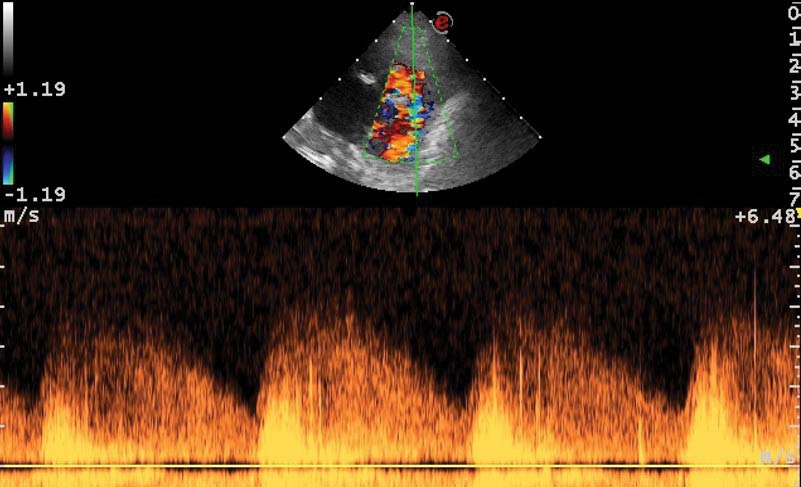
This vessel diameter was greater than the right and left branches of the main pulmonary artery, with an ampulla measuring greater than 10mm and an ostium between 4.5mm and 5mm. The left atrium was severely dilated, with a left atrium to aorta ratio of 2 (reference less than 1.5).
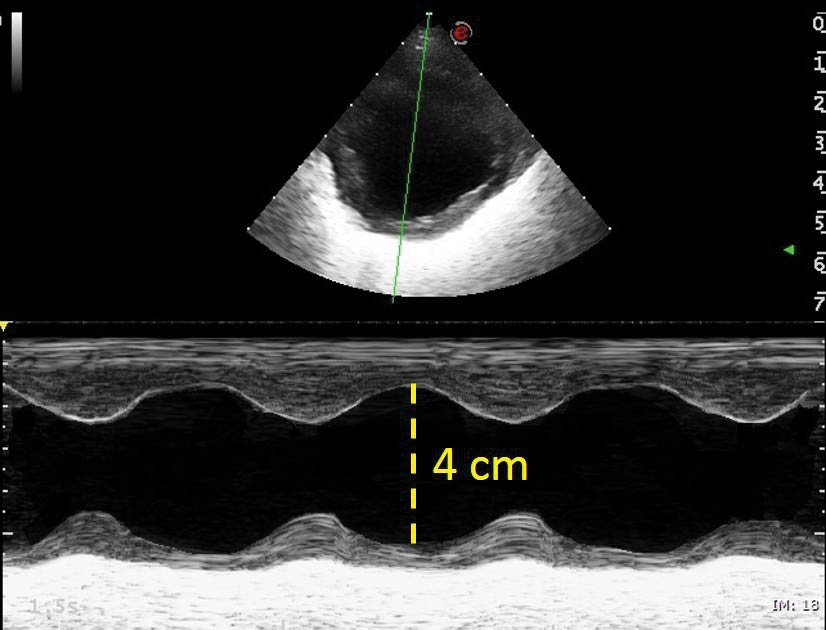
The left ventricle appeared rounded, dilated and hyperkinetic, measuring more than 4cm in diastole in short axis and with a fractional shortening of 48% (reference less than 25%; Figure 3).
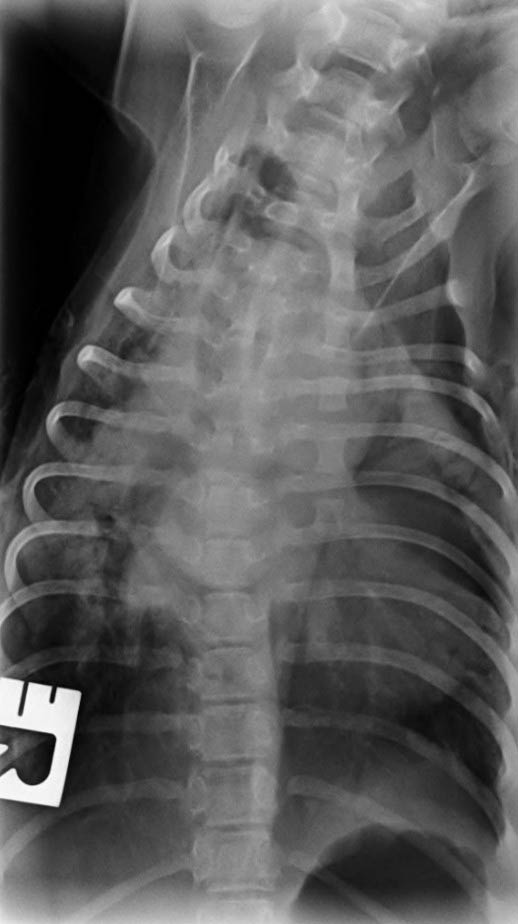
Two consecutive boluses of furosemide at 2mg/kg and a single dose of pimobendan at 0.3mg/kg were administered IV and flow–by oxygen was provided via face mask. One conscious dorsoventral thoracic radiograph was obtained to confirm the presence of severe cardiomegaly and a generalised interstitial pattern, and presence of extensive perihilar pattern, consistent with severe pulmonary oedema (Figure 4).
The dog was admitted to the intensive care unit to receive hourly furosemide boluses and continuous monitoring of the heart and respiratory rates, as well as the systolic blood pressure.
By late afternoon, the dog appeared to be doing much better, with improvement in mentation, reduction of pulmonary crackles on auscultation, elevation of systolic blood pressure from 60mmHg to 120mmHg and improvement in mucous membrane colour. However, at about 6:30pm, it deteriorated acutely and became dyspnoeic and tachypnoeic again.
At this stage, extreme respiratory muscle fatigue was a major concern, so induction of general anaesthesia to perform positive pressure ventilation was discussed with the owner with an aim to improve blood oxygenation levels and reduce pulmonary oedema.
It was also discussed that, as the risk of an anaesthetic procedure was going to be assumed – and to relieve the volume overloaded failing left-sided heart – we could attempt to occlude the PDA during the same anaesthetic procedure.
To avoid thoracotomy, ideally the PDA would be closed minimally invasively with a canine duct occluder (CDO) device, as this usually allows improved postoperative recovery with minimal analgesia requirement. However, the dog’s femoral artery was assessed ultrasonographically and had a diameter smaller than 2mm (smaller than the diameter of the catheter required for the deployment of the CDO device).
Also, the echocardiographic appearance of the PDA was suspicious of a type C morphology (Schneider, 2003); this is a ductus that has a tubular shape that does not taper significantly at the level of the ostium and, therefore, cannot hold the implantable device in place.
As a consequence, it was decided – after time under forced ventilation, to improve the oxygen saturation of the circulating blood – thoracotomy would be performed to surgically ligate the PDA.
The dog was positioned in right lateral recumbency and thoracotomy was performed over the left fourth intercostal space. Cautious extra-pericardial dissection of the PDA revealed its external morphology to be very wide, but short, with minimal space to pass the ligature around the ductus. However, after careful dissection, it was successfully ligated with 2/0 silk and 3/0 nylon sutures. Standard chest drains were considered too large for the size of this patient, so an 8G feeding tube was temporarily placed to function as a drain until the chest was closed when it was subsequently removed.
During anaesthesia, the dog was placed on a mechanical ventilator with 40% oxygen, which continued for 90 minutes post-procedure (Figure 5), along with a constant rate infusion of furosemide at 1mg/kg/h.
Recovery from surgery was uneventful and, over the following 24 hours, the dog was gradually weaned off diuretics, analgesia and oxygen completely. He became mildly hypokalaemic, which was resolved with slow IV infusion of potassium and once he began to eat.
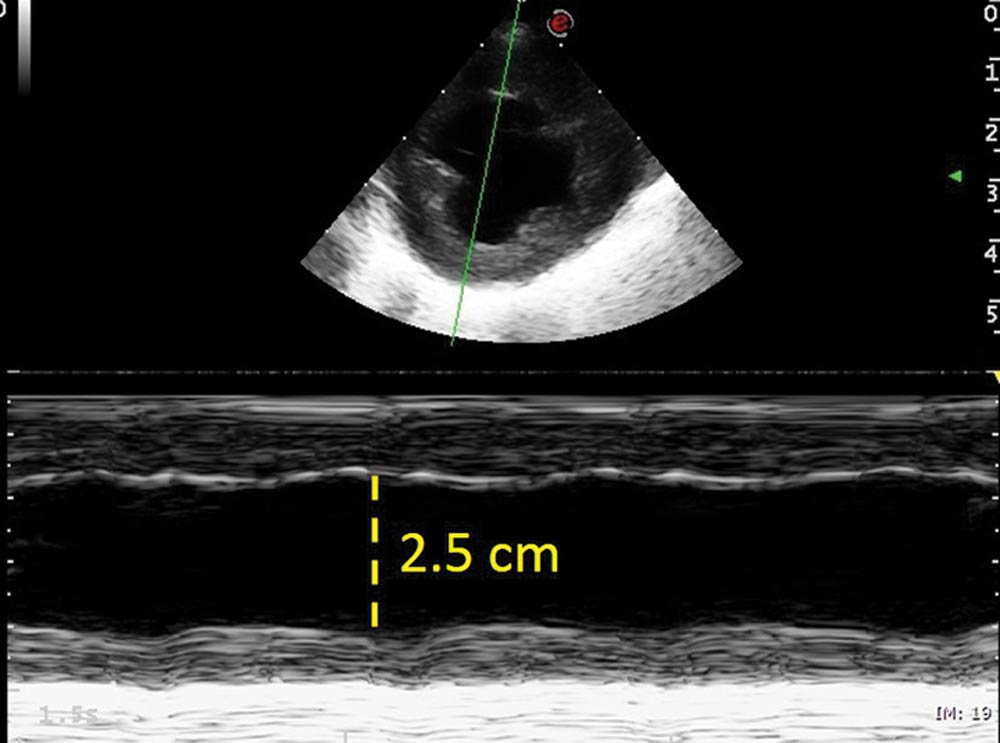
A repeat echocardiogram the following day showed much improved cardiac dimensions, with the left atrium to aorta ratio now at 1.3 and the left ventricular dimension in diastole at 2.5cm (Figure 6). A mild mitral regurgitation jet, due to annular dilation, was still present and the contractility appeared markedly reduced, with the fractional shortening lower than 20%. This apparent reduction of contractility is a normal finding after PDA ligation, although it may recover and return to normal in such a young animal with early intervention.
The puppy was discharged the following day with no medication and strict cage rest for the following three weeks to allow its wound to heal (Figure 7). One year later, the dog continues to do well and, for this reason, the owners declined performing any further diagnostic tests, including a follow-up echocardiogram.
The heart is the first functional organ to form in the fetus and begins to beat on day 18 to day 19 of gestation in the dog and day 22 in humans. During fetal development, the circulatory system is a single closed circular circuit. Oxygenated blood (haemoglobin saturation of about 85%) comes from the placenta and 50% of it is diverted through the ductus venosus, bypassing the underdeveloped liver.
Blood arrives through the sinus venosus to the right atrium, where a large proportion is diverted through the foramen ovale directly into the left ventricle. The small amount of blood that does not cross through the foramen ovale to the left atrium will go from the right atrium to the right ventricle and from there to the non-ventilated, collapsed lungs.
The left ventricle, therefore, receives well-oxygenated blood, but it is also mixed with a small amount of blood with low oxygen content coming back from the lungs through the pulmonary veins. From the left ventricle, oxygenated blood (65% saturation) goes to the ascending aorta and, from there, to the head and forelimbs, through the brachiocephalic trunk and left subclavian artery. After this, poorly oxygenated blood from the pulmonary circulation will cross the ductus arteriosus towards the descending aorta to produce mixed oxygenated blood that will go to the lower body and back to the placenta.
At birth, the three communications (that is, ductus venosus, foramen ovale and ductus arteriosus) need to close. After this postnatal change in circulation, the mature circulation consists in two parallel circuits connected in series.
Changes in prostaglandin concentrations from the mother’s placental circulation and different oxygen concentration will be the stimuli that will cause the vascular smooth muscle of the ductus to contract, collapse and close its circulation during the first hours to days after birth. If this does not happen, it will leave a patent ductus arteriosus (PDA).
Left-to-right PDAs cause volume overload on the chambers that receive the excess of blood, including the pulmonary artery, lung vessels, left atrium, left ventricle and ascending aorta. The amount of shunted blood through the ductus will determine the severity of the volume overload and, therefore, the severity of clinical signs.
According to Schneider’s angiographic descriptions adapted from a human classification, up to five types of PDA exist depending on the vessel’s shape (Schneider et al, 2003). From these, the ones that appear to cause the larger amount of shunted blood are likely to be the ones with a tubular shape (type C), but any of the other types can eventually also cause early signs of congestive heart failure if enough blood is shunted through the short circuit.
Treatment of severe acute congestive heart failure signs is directed at alleviating hypoxia with aggressive therapy, including oxygen and forced diuresis, while providing intense monitoring. The oxygen requirement to sustain ventilation in healthy individuals is less than 5% of the total oxygen delivery. However, during an acute crisis – and because of the increased work of breathing – these requirements may increase to more than 25%. Moreover, decreased lung volume induces alveolar collapse while hypoxia induces pulmonary vasoconstriction, worsening the blood oxygen saturation even further.
This increased oxygen requirement to sustain ventilation, in addition to the compromised oxygen delivery to other organs caused by the primary cardiac condition and the reduced alveolar oxygen uptake caused by pulmonary oedema, will worsen the cardiorespiratory status of the patient over time. This may lead to fatal respiratory fatigue and respiratory muscle failure, leading to respiratory death, even before cardiac arrest.
Mechanical ventilation is indicated when an adequate spontaneous gas exchange can no longer be maintained (Hopper and Powell, 2013). The two major uses for positive pressure ventilation via mechanical ventilation are hypoxaemia refractory to conventional therapy and ventilatory failure; severe congestive heart failure cases may suffer from a combination of both situations.
Mechanical ventilation decreases the work of breathing, resulting in an increased oxygen saturation and delivery to all organs (Pinsky, 2005). Consequently, overall organ function, but specifically myocardial function, improves, allowing for more efficient cardiac function.
Furthermore, positive-pressure ventilation causes an increase in the intrathoracic and intra-abdominal pressure, which causes a reduction in the venous return and reduction on the filling of the right-sided chambers – which, in patients with pulmonary oedema, may be beneficial in helping recruit interstitial water back into the vessels. Similarly, withdrawal of ventilatory support in patients with limited cardiovascular reserve should be done slowly because the increased load on the heart can precipitate heart failure and pulmonary oedema.
Recognising severe respiratory muscle fatigue and determining the right time to induce anaesthesia to provide mechanical ventilation in a severely compromised patient is always difficult.
However, like in the presented case, an initial response with improvement in clinical signs (reduction of the respiratory rate) followed by sudden deterioration should suggest sudden oxygen reduction is the likely cause and urgent measures need to be taken.
Fortunately, for this case, it was possible to fully and successfully correct the underlying cardiac disease responsible for the pulmonary oedema and, as such, completely revert the cardiorespiratory compromise back to a normal physiological state.