10 May 2021
Karen Perry discusses two commonly encountered causes of lameness in cats, and evaluates the experience and literature base used to direct diagnosis and treatment.

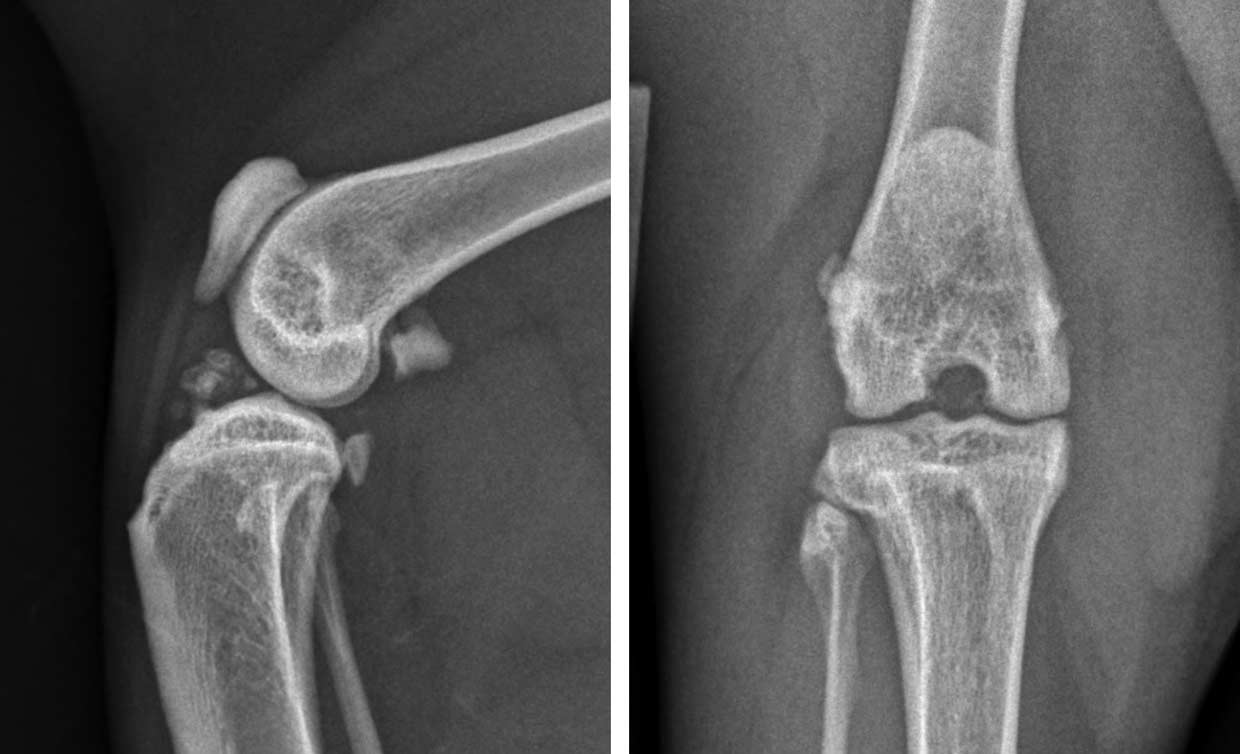
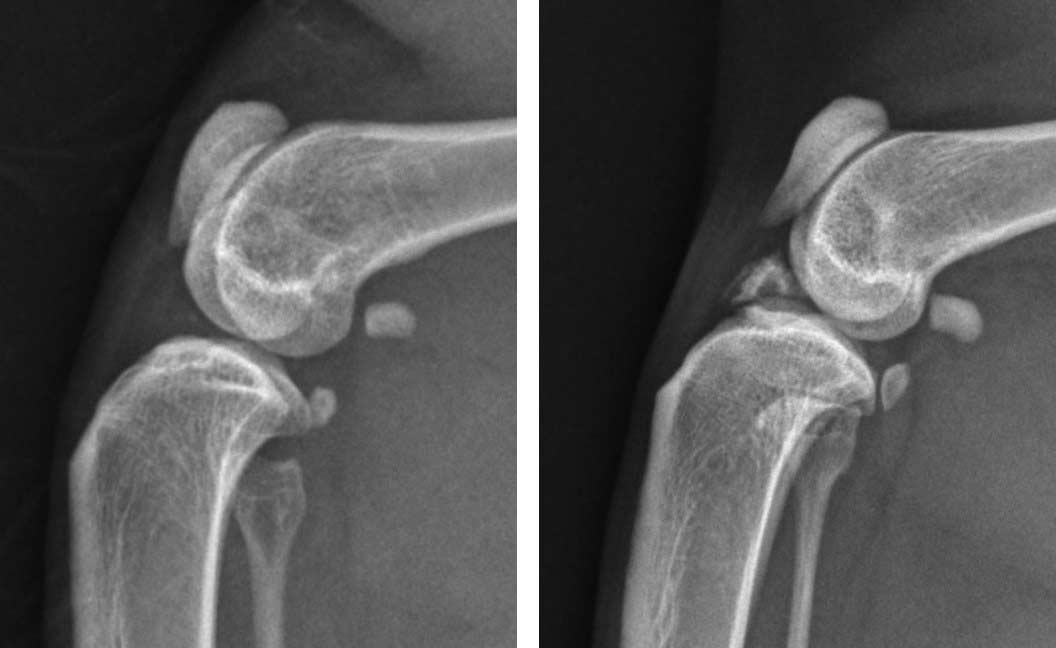
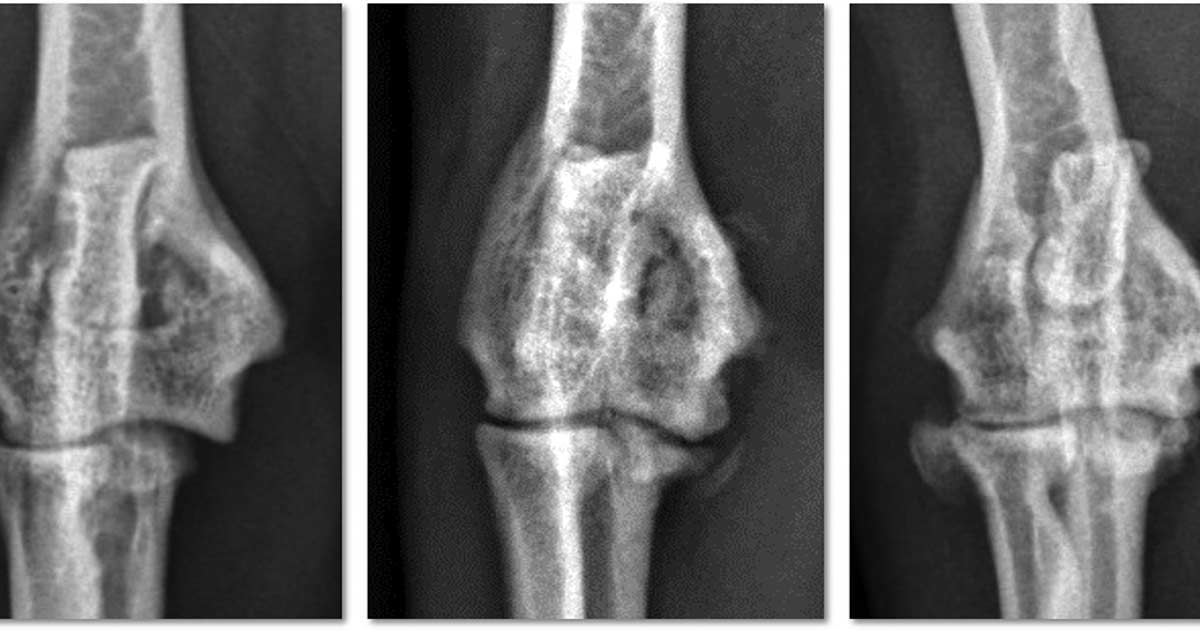
Figure 1. Craniocaudal views of the elbow of three cats, all diagnosed and treated for medial humeral epicondylitis. Left: radiograph of a cat in the earliest stages of the condition with minimal radiographic change; the definitive diagnosis was made via ultrasound. Centre: radiograph of a mild, chronic case with rounding and irregularity of the medial humeral condyle. Right: radiograph of a moderate-to-severe chronic case with bony spur formation over the medial humeral condyle.
This article focuses on two commonly encountered causes of lameness in cats, and evaluates the experience and current literature base used to direct their diagnosis and treatment.
Medial humeral epicondylitis is a common cause of forelimb lameness, which can be challenging to diagnose. Cats with an active outdoor lifestyle appear to be at increased risk for developing this condition, which is suspected to be an overuse injury. While severe and chronic cases are readily diagnosed via radiography, once the condition progresses to this stage, the prognosis for resolution of lameness without surgical intervention is poor.
The potential role of ultrasound to facilitate an earlier diagnosis is discussed, which may render cats more likely to respond to non-surgical management, including lifestyle changes and physical rehabilitation.
Feline cranial cruciate ligament failure represents an important cause of hindlimb lameness of uncertain aetiology; the respective roles of trauma and degeneration remain hotly debated in this species. While a diagnosis is generally simple to achieve based on characteristic clinical signs and radiographic findings, the ideal treatment for this condition remains controversial. The potential roles for non-surgical management, static stabilisation and dynamic stabilisation are discussed.
This article will cover two commonly encountered causes of lameness in cats, and evaluate the current evidence base that guides their diagnosis and treatment.
Medial humeral epicondylitis (MHE) is a relatively frequent cause of forelimb lameness, which can be challenging to diagnose and treat. Cranial cruciate ligament (CrCL) failure, on the other hand, is generally readily diagnosed following an orthopaedic examination, but recommendations regarding treatment vary widely, which can complicate decision-making.
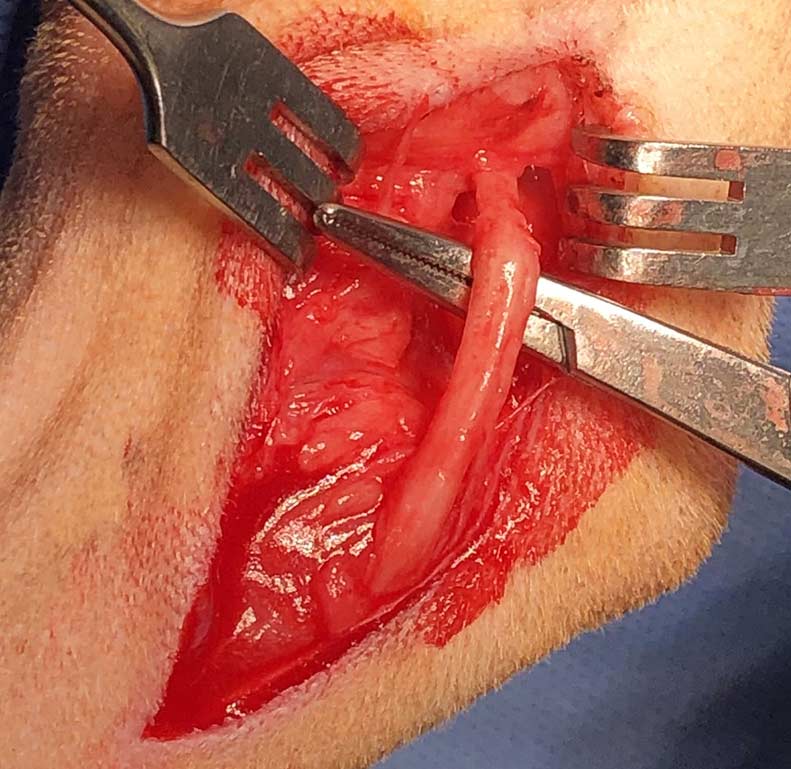
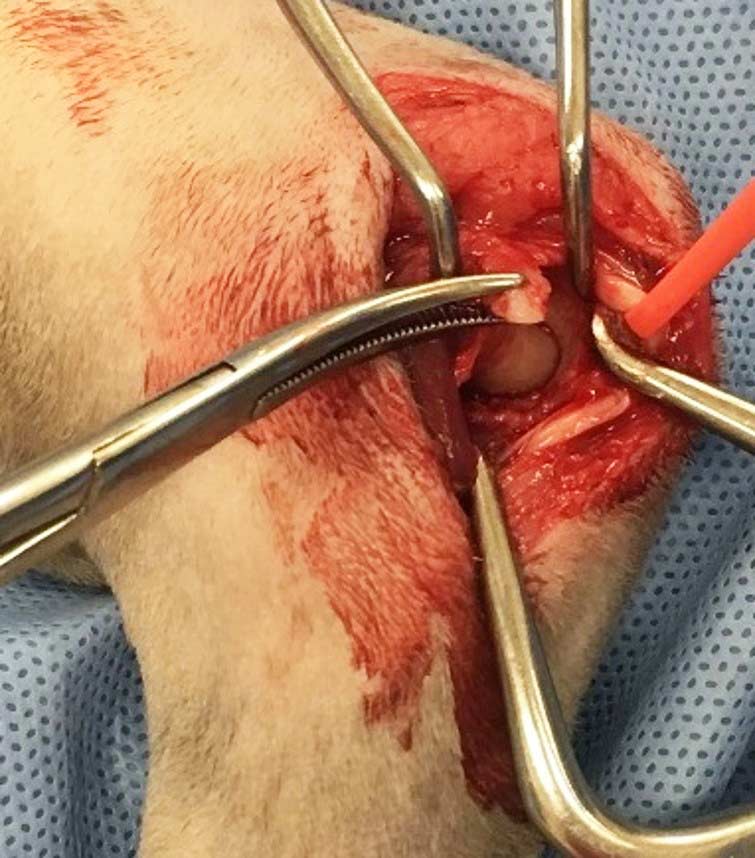
MHE is a cause of elbow lameness in cats, which has been reported in up to 10% of cats based on postmortem examination. The condition is characterised by chronic degeneration, mineralisation and metaplastic new bone formation within the antebrachial flexor tendons at their insertion site on the medial epicondyle of the humerus. The humeral head of the flexor carpi ulnaris muscle, which is closely connected to the joint capsule as it surrounds the medial epicondyle, is the most commonly affected tendon (Streubel et al, 2012).
The cause of MHE is essentially unknown, but may be related to repetitive trauma or overuse; strains and traumatic avulsions of the tendons of origin of the flexor muscles lead to tendinosis, partial tearing and sometimes full-thickness tears of the tendons (Streubel et al, 2012; Voss et al, 2009; Nirschl, 1992). The bony spurs and free fragments associated with MHE can also cause separation of the humeral and ulnar heads of the flexor carpi ulnaris. The ulnar nerve is confined between these muscles – allowing aberrant mineralisation to impinge the nerve, causing flattening and displacement, adhesions and epineural fibrosis (Streubel et al, 2012).
The reason why the humeral head of the flexor carpi ulnaris muscle is predominantly affected by MHE remains unclear. The flexor carpi ulnaris has been reported to be the most important antigravity carpal flexor during stance and locomotion (Glenn and Whitney, 1987) and the humeral head contains more slow postural muscle fibres than any other feline forelimb muscle (Goneya et al, 1981). These factors may explain the susceptibility of this particular muscle to repetitive trauma or overuse injury. Additionally, the anatomical position with a separate insertion on the accessory carpal bone may predispose to overuse trauma (Streubel et al, 2015).
Cats with MHE can present with and without concomitant intra-articular changes (Streubel et al, 2012). In a postmortem study of six cats with MHE, immediate postmortem radiographs revealed mild-to-moderate subluxation of the humeroulnar or humeroradial joint in three cats (Streubel et al, 2012), and it was postulated that the thickened and partially mineralised joint capsule led to subluxation of the humeral condyle and the subsequent development of cartilage defects. The clinical significance of this subluxation remains to be elucidated.
In one study evaluating clinically affected cats with MHE, no mention occurred of subluxation affecting any cat (Streubel et al, 2015). However, a recent case report postulated that the same joint capsule thickening responsible for this subluxation may also result in collateral ligament instability in some cats with MHE (Perry, 2017).
Reports of CrCL rupture in cats are remarkably less frequent than in dogs (McLaughlin, 2002). One reason for this may be that the CrCL is larger than the caudal cruciate ligament in cats, whereas the reverse is true in dogs (Harasen, 2005; Umphlet, 1993; Kunkel et al, 2009). Another potential explanation may be the lower amount of differentiation of fibrocartilage when compared with the dog, which has been proposed to be due to the relatively lower body weight of cats in general (Wessely et al, 2017). It is also possible that a proportion of cats with CrCL rupture are treated conservatively and never presented for evaluation (Harasen, 2005).
Despite the reduced frequency, it is recognised that feline CrCL rupture certainly does occur, likely with greater frequency than is reported, and that when it does occur it can result in medial or lateral meniscal tears (Ruthrauff et al, 2011), stifle instability (Maitland et al, 1998) and secondary OA (Boyd et al, 2005). These changes result in stifle pain, lameness and joint effusion (McLaughlin, 2002) and, as such, warrant treatment.
While the exact aetiology of canine CrCL disease remains undefined and controversial (Comerford et al, 2011; Brunnberg, 1989), data from the collective literature base suggests that various factors can promote degeneration of the CrCL and increase the risk of developing CrCL disease (Witsberger et al, 2008; Whitehair et al, 1993).
The histological changes that have so far been implicated in CrCL disease are degeneration and chondroid metaplasia of the extracellular matrix, decreased ligamentocyte density, disorganisation of collagen fibres and phenotypic changes in ligamentocytes (Ichinohe et al, 2015). However, dogs normally begin to show signs of loss of the typical ligament structure at an early age (two to five years old) and the presence of shattered or ruptured collagen fibres is common (Reese, 1995). Specific signs of degeneration within the fibrocartilage cells, marked cartilage substance and cartilage cells with well-demarcated capsules are also observed regularly (Reese 1995).
It is unclear whether the pathogenesis of CrCL rupture in cats is similar to that in dogs, or if feline patients are unique in that trauma plays a much greater role in the disease process (Ruthrauff et al, 2011). While a traumatic event is often assumed to be the main cause for CrCL rupture in cats (McLaughlin, 2002; Tacke and Schimke, 1995), the actual cause is not clear in every case (Harasen, 2005; Matis et al, 2010).
In one study (Wessely et al, 2017), 78.9% of cats had sustained a traumatic event, while the remaining 21.1% of cats all resided indoors exclusively and had no evidence of trauma.
However, reports vary in terms of how often a traumatic event is reported. While Tacke and Schimke’s study (1995) reported a similar percentage of traumatic cases with 80% of cats having a history of trauma, Scavelli and Schrader (1987) reported that only 16% of the cats in their study had a clear traumatic event, with the cause of rupture remaining unknown for 84%.
A proportion of cats have been proposed to be affected by degeneration of the CrCL that may precede rupture. This postulation stemmed from the subset of feline patients suffering from CrCL rupture noted to mirror the older, overweight, small-breed canine patients in which degenerative rupture is seen (Harasen, 2005).
A report from Harasen (2005) provided data from histopathological examination of one feline CrCL with a non-traumatic rupture, and for a long time this represented the single publication providing insight into CrCL rupture in cats and indicated that degeneration may play a role in aetiopathogenesis.
Subsequent studies have added further strength to this argument. Two studies have reported that some cats with CrCL rupture have pre-existing radiographic OA at the time of presentation, which would render a degenerative cause more likely (Voss et al, 2017; Mindner et al, 2016). Histopathological evaluation has also been reported that demonstrates degenerative changes present in feline CrCL prior to complete rupture (Voss et al, 2017).
Another finding that potentially indicates solely traumatic injuries to be less likely was the recent report that 14% of cats develop bilateral disease, with the mean interval between injuries being 18.8 months (Boge et al, 2020). While this is substantially less than the frequency of bilateral disease typically reported in canine studies (Grierson et al, 2011; Moore and Read, 1995; Harasen, 1995; Buote et al, 2009; Mölsä et al, 2013; Muir et al, 2011), it still appears to be a substantial number if the aetiology were solely traumatic.
The aforementioned evidence supporting a degenerative cause of CrCL in cats appears compelling; however, the issue remains controversial as another histopathological study (Wessely et al, 2017) failed to confirm any evidence of a degenerative cause in cats. Further research is required in this area before firm conclusions regarding the aetiology of CrCL failure in cats can be made.