29 Apr 2019
Hany Elsheikha discusses why interdisciplinary partnerships should be considered the way forward in mitigating the risk of infection faced by humans from cats and dogs.
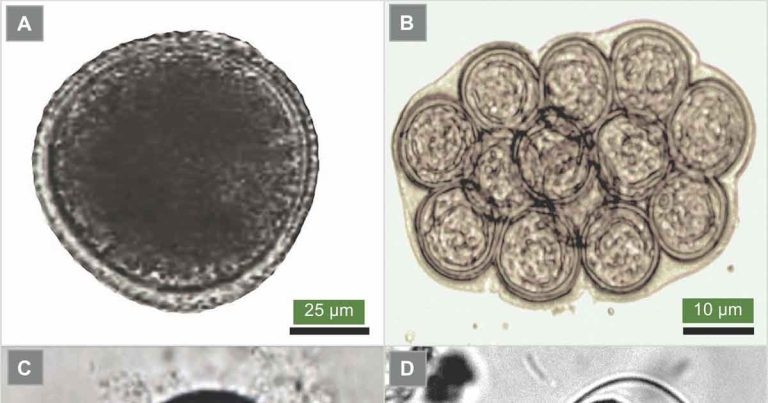
Figure 1. The eggs of Toxocara canis (1a), the common roundworm of dogs; Dipylidium caninum (1b), the common intestinal tapeworm of dogs and cats, also known as flea tapeworm; Echinococcus species (1c), the dwarf tapeworm; and the dog hookworm Ancylostoma caninum (1d).
The need for multidisciplinary collaboration to tackle today’s complex health challenges caused by parasitic infections has never been more important.
Parasitic infections – especially where animal and human health are linked – present a major challenge to veterinary and medical professionals, as well as public health officials. These challenges have been compounded by rapidly shifting trends of human behaviour and globalisation. As the burden of parasitic zoonoses continues, pressure is mounting on the global community to find new tools and innovative solutions for management of these infections.
The one health approach represents a true opportunity for all professionals, from all health care disciplines, to jointly develop interdisciplinary strategies to control zoonotic parasitic diseases that compromise the health and welfare of companion animals and humans.
This article will discuss how one health can offer solutions for tackling threats caused by zoonotic parasitic diseases. It will also emphasise the importance of establishing more cross-sector collaboration between professionals from veterinary medicine, human medicine, public health, and other related fields.
The important role of dogs and cats in people’s lives, as companions, is well established. Many health-enhancing effects to humans exist from such interactions – including improvements in physical, social and mental health.
On the other hand, the health of dogs and cats is intimately linked with that of their owners – and they share the same risk of exposure to parasite infection. This close contact with dogs and cats, their ability to serve as sentinels for – or sources of – human infection, and the rapid socioeconomic and demographic changes in some societies can increase the spread of parasitic zoonoses from pet animals to humans (Macpherson, 2005).
Generally, all household members are at risk of acquiring parasitic infections. However, immunosuppressed people are at greater risk of infection from animal-borne zoonotic parasites. These at-risk groups include pregnant women, newborns, patients with various types of cancers, organ transplant recipients, AIDS patients, and patients undertaking a long course of treatment with immunosuppressive drugs.
Parasitic zoonoses have gained increasing national and international attention in recent years, owing to their adverse impact on animal and human health. A neglected or incorrect diagnosis, or delayed treatment, will cause severe health consequences. When it comes to animal and human health matters, society has become more proactive than ever.
Over the past few decades, a substantial effort has occurred in developing means for detection, treatment and control of parasitic diseases in pets and humans. However, despite this progress, parasitic diseases remain a major threat in many parts of the world.
Many factors make optimal control of parasites, especially zoonotic species, difficult to achieve. A key factor contributing to this complexity is the ability of some parasites to transform into different life cycle stages as they transfer through numerous hosts (domestic and wild animals) to ensure their survival. To tackle this challenge, adopting a one health approach to promote interdisciplinary collaboration is more important than ever.
This article will briefly outline how parasites have a complex biology and measures of controlling some of the most challenging parasite infections – presenting echinococcosis and leishmaniosis as examples. It will also discuss why an interdisciplinary one health approach can be considered the way forward for mitigating the risk caused by zoonotic parasitic infections.
Companion dogs and cats can acquire worm infections through various modes of transmission – such as contaminated soil (for example, Toxocara canis; Figure 1a), accidental ingestion of fleas (for example, Dipylidium caninum; Figure 1b), and ingestion of snails and other gastropods (for example, lungworms Angiostrongylus vasorum [dogs] and Aelurostrongylus abstrusus [cats]).
Additionally, dogs and cats can become infected through scavenging and hunting (for example, the tapeworms Echinococcus granulosus and Echinococcus multilocularis; Figure 1c); or via placental transmission from the mother to the fetus, or mother’s milk to newborn puppies (for example, roundworm T canis and hookworm Ancylostoma caninum; Figure 1d).
All of these parasites can also infect humans, through ingestion of fruit and vegetables contaminated with eggs passed in the faeces of dogs and cats (for example, T canis or Echinococcus species). Zoonotic parasite infection can also occur transdermally (for example, A caninum). It is also possible humans – especially children – become infected with D caninum if they accidentally ingest fleas containing the infective cysticercoid larvae.
In-depth description of zoonotic parasitic diseases transmitted to humans from companion dogs and cats is beyond the scope of this article. Readers are encouraged to consult some important articles (Fisher, 2001; Fisher, 2002; Ridyard, 2005; Helm and Morgan, 2017), guidelines (for example, the European Scientific Counsel Companion Animal Parasites Guidelines) and textbooks (Elsheikha et al, 2018) for more information.
Although most parasites complete their life cycle inside a single host, some – particularly those involving humans – have complex life cycles where they are transmitted across multiple hosts.
It is unclear what favours this complex style of parasite biology – or whether any advantage exists of adopting such a complex lifestyle, and the long-term consequences of cycling within multiple hosts. Nonetheless, these complex life cycle parasites are often the ones that cause serious diseases in animals and humans.
Therefore, to better understand the public health and economic impact of infection with complex life cycle parasites – and to improve treatment and preventive programmes – it is important to consider the health effects of these parasites across all animal species they cycle through.
Emerging infectious diseases have always been considered a serious threat to both human and animal health. About 75% of emerging infections are zoonotic (transmitted from animals to humans), of which 70% originate in wildlife populations.
Therefore, control of zoonotic parasitic infections should not stop at treating humans only; management of infection in companion animals and wildlife is also important, and should be considered.
Companion and wildlife species play roles in maintaining some of the most devastating parasitic diseases. Surveillance of the concerned animal species for zoonotic parasites is an important component of a one health strategy to determine the possible transmissibility to humans and pets, and effectively respond to – and mitigate – the potential adverse impacts of likely or imminent emerging zoonotic diseases.
Without considering limiting or blocking the transmission of infection from the reservoir animals to humans, an optimal parasite control strategy may never be achieved.
Echinococcosis is caused by larval stages of the tapeworms E granulosus and E multilocularis.
E granulosus is maintained in nature by cycling through definitive hosts (for example, dogs and cats) and intermediate hosts (for example, cattle, sheep and goats).
Dogs can harbour hundreds of these tiny tapeworms without exhibiting particular clinical signs. Humans can be infected accidentally by ingestion of food or water contaminated with dog faeces, which leads to a disease called cystic echinococcosis, which is endemic in Europe. In the UK, cystic echinococcosis is prevalent in the sheep farming communities of central and southern Wales.
In humans, E granulosus forms hydatid cysts mainly in the liver and lungs. Other parts of the body – such as the brain, heart and bones – are less frequently affected.
Cysts may remain asymptomatic for years and vary in size considerably. Large cysts can cause organ dysfunction due to the mass effect. Rupture of hydatid cysts – accidentally or during surgical interference – causes an allergic reaction to parasite antigens.
The fox tapeworm E multilocularis has a sylvatic life cycle that involves a broad range of host species. Foxes, wolves and coyotes serve as predator definitive hosts, while the prey intermediate hosts involve voles, lemmings, shrews and mice. This parasite can spill over into dogs – and sometimes cats – which harbour this tapeworm in their intestine, where they cause very limited harm for the infected animal.
Human infection with the fox tapeworm leads to a disease known as alveolar echinococcosis, which can cause serious health consequences.
E multilocularis tapeworm behaves like a tumour and spreads throughout the body of infected humans. Without early recognition of infection and timely administration of effective treatment, infection can cause up to 75% mortality rate and higher in individuals with limited pre-existing immunity. For these reasons, infection with E multilocularis in humans has been given a high priority compared to companion animals.
However, echinococcosis in humans and animals should not be considered as separate problems – in fact, control of echinococcosis disease in dogs, and limiting exposure to reservoir animals, are a prerequisite for effective infection control to protect the public health.
Alveolar echinococcosis is inflicting a major impact on the health systems in some European countries (for example, Switzerland), probably due to a rise in the number of infected foxes, the main host for the parasite in Europe. Fortunately, E multilocularis is not endemic in the UK; however, increase in pet travel – and the relaxation in the time allowed between compulsory tapeworm treatment and return to the UK – make introduction of this fox tapeworm without preventive measures highly likely (Torgerson and Craig, 2009).
The Pet Travel Scheme requires dogs to be treated with praziquantel between one and five days before entering the UK. This has prevented E multilocularis from establishing in the UK. However, the prepatent period of fox tapeworm is at least 30 days; therefore, pet owners should treat dogs monthly while visiting endemic countries to prevent excretion of eggs that would put dogs at immediate risk.
The half-life of praziquantel is approximately 12 hours, so infection may occur in the 5-day period between compulsory treatment and entry to the UK. Therefore, all travelled dogs should receive an additional praziquantel treatment within 30 days after entry to the UK.
This zoonotic disease – caused by Leishmania infantum – affects humans, dogs and cats. The dog is the main domestic reservoir and can serve as a sentinel for Leishmania infection in humans. However, a number of wild mammals (for example, golden jackals, Iberian lynx, mustelids, red foxes, wildcats and wolves) have been found infected.
Leishmaniosis is not endemic in the UK and infection is acquired abroad because transmission occurs mainly through sandfly bites (Figure 2). However, in an era of increased foreign travel of dogs – and increased influx of potentially infected “trojan” rescue dogs to the UK (that are seropositive for L infantum) – leishmaniosis has become an important health challenge in the UK. It does not take more than one imported sandfly to establish the disease in a new geographic locality (Wright and Baker, 2019).
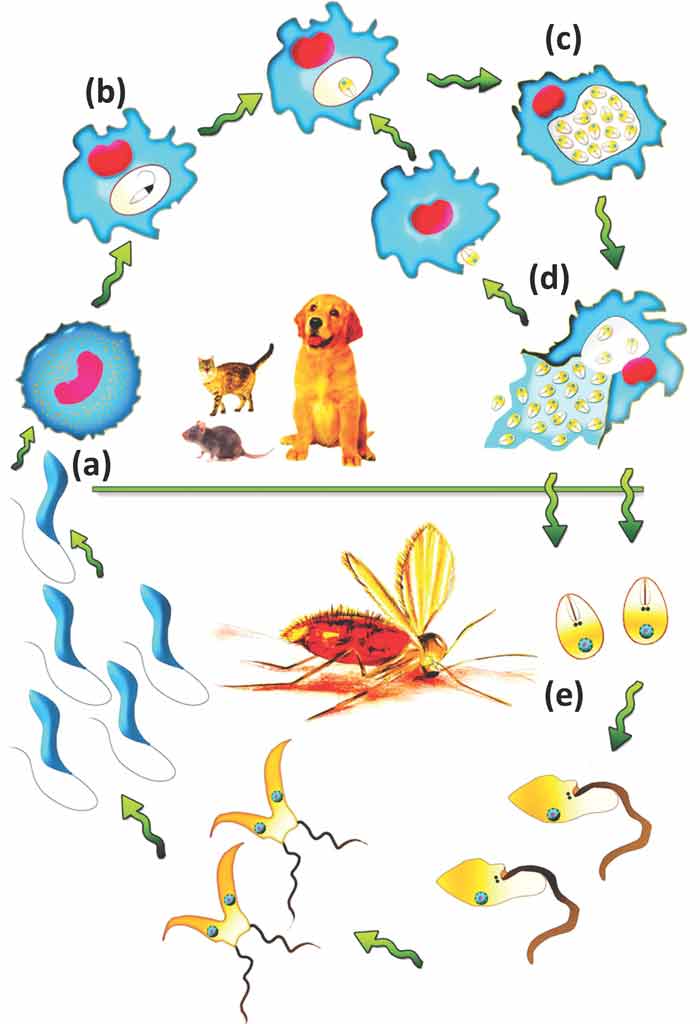
Additionally, Leishmania-infected dogs may present an infection risk to other dogs, as direct transmission between dogs is possible (Karkamo et al, 2014; Daval et al, 2016).
Canine leishmaniosis signs include alopecia, dermatitis, polyarthritis, uveitis, hepatopathy, glomerulonephritis, and neurological signs. For a suspected case, it is important to consider compatible clinical signs combined with common epidemiological features – such as travel to an area endemic for leishmaniosis or residence in such an area, and/or history of sandfly bites. Therefore, travel history information should involve more than asking merely for the name of the country a patient has been to.
Also, in light of the development and detection of the first case of leishmaniosis in the UK in a dog without a travel history to an endemic area (McKenna et al, 2019), we need to be extra-vigilant to guard against the establishment of this infection in the UK. Therefore, even in the absence of travel history – and in the absence of the natural vectors – we need to remain cognizant that direct transmission between dogs can occur through bites or wounds.
Preventive measures are vital for the health of dogs and cats travelling to endemic countries, because this disease can be chronic, and may require a long-term serological monitoring and repeated treatment.
Vaccination can be offered for dogs travelling to endemic regions, such as southern European countries. One vaccine uses parasite antigens to promote a cell-mediated response and confers reasonable protection against clinical disease; however, vaccination cannot prevent infection, so repellents should be used in addition to vaccination.
Licensed products containing pyrethroids are available in the UK as spray, spot-on or collar formulations for use in dogs. Also, a collar containing flumethrin and imidacloprid is licensed to reduce the risk of infection with Leishmania via transmission by sandflies for up to eight months.
Treatment using collars should be considered one week before travelling to endemic areas – or at the beginning of the vector season for dogs living in endemic regions – to ensure enough time for the insecticide to reach full distribution and activity over the dog’s skin. Spot-on formulations should be applied at least two days prior to exposure, to ensure animals are protected.
For years, the scientific community has been discussing the concept of one health, and how it could help tackle the challenges caused by infectious diseases and outbreaks.
The growing interest in adopting such an interdisciplinary approach to developing more effective strategies to control zoonotic infections has been evolved in response to many variables – such as the shift in people’s demands of better health care services, especially among millennials.
For several decades, universities have been playing a huge role in training animal and human health care providers; however, a need still exists to dissolve the historical divide in the perspectives of academicians and clinicians.
On the one hand, research-led teaching is what basic scientists believe is the most appropriate learning approach. On the other hand, clinicians believe hands-on clinical teaching is the most valuable way of teaching health professionals.
It is time to address the gaps in the university curriculum to make it more integrated – by considering inputs from clinicians who have patient experience, and basic scientists equipped with latest scientific knowledge. Establishing creative and clinically oriented learning strategies will ensure more interdisciplinary training of future generations of health care providers.
Moreover, involving specialists from the industry in curriculum design will bridge the gap between knowledge producers and knowledge users. A need exists to find new ways of creating empowered partnerships among physicians, veterinarians, pet owners, environmental health, policymakers and drug companies to jointly develop and test new interventions. These partnerships are needed if we are to effectively reduce the risk of parasitic diseases in companion animals and humans.
“If at first the idea is not absurd, then there is no hope for it.”
Albert Einstein
Having health care professionals who have not traditionally worked together in the same clinic – integrating their expertise to deliver health services essential for the management of diseases in humans and pets – is absurd, but definitely an outside-the-box idea.
Knights Landing One Health Center, in California, US, is the first one health-based clinic to be established based on an interdisciplinary operational model where veterinarians and physicians work side by side. This interprofessional closeness has facilitated knowledge exchange between both professions, and enabled the delivery of a better health care service for humans and pets in the local community (Sweeney et al, 2018).
Another study has explored how human health care and veterinary professionals’ experience differs in respect to management of leishmaniosis. It found many lessons from both fields could be integrated and put into practice (Miró and López-Vélez, 2018).
Clear advantages exist of fostering working relationships between vets and physicians. Creating such an integrated health care service model is integral to the success of any parasite management strategy.
Significant governmental resources are being used for running efficient health care systems to improve the health of humans. However, the growing threats of emerging and zoonotic infections call on governments for more commitment and attention to building more multidisciplinary (one health) strategies, and providing more support for innovative emerging solutions.
Implementation of a one health approach for infection control should not be left only to local communities and professional organisations. More financial support is needed to promote interdisciplinary collaboration, and enable health care professionals to gain an appropriate level of knowledge and skills to effectively control zoonotic infections.
Prevention of parasitic infections in pets and humans should be an important priority. Considerable progress has been made over recent decades, in terms of development of better diagnostic techniques and optimisation of treatment regimens; however, infection rates are still unacceptably high, so more work is required.
The persistence of parasitic diseases highlights the need for better partnerships and collaborations across disciplines. Progress in parasite control will depend largely on the production of knowledge derived from integrated programmes of fundamental and applied research. Effective use of this knowledge cannot be achieved without sharing information and combining expertise across disciplines.
Vets and physicians need to establish effective working relationships, and share their different perspectives of patient care; one profession cannot do it alone.