12 Jul 2022
Laura Bree outlines initial symptoms of this condition in cats and dogs, helping vet surgeons spot it before it develops.

Image © New Africa / Adobe Stock

Chronic kidney disease (CKD) is diagnosed based on the presence of combined azotaemia (increase in creatinine concentration) and/or inadequately concentrated urine.
The history and clinical findings can vary depending on the aetiology of CKD. The classification system by the International Renal Interest Society (IRIS) was designed to offer a standardised and widely accepted staging system for CKD, to guide treatment and prognosis for dogs and cats with CKD.
The first stage of CKD pertains to non-azotaemic patients – that is, creatinine concentrations less than 125μmol/L in the dog and less than 140μmol/L in the cat, or symmetric dimethylarginine (SDMA) concentration less than 18μg/dL (Figure 1).
These patients are either considered at risk of renal disease or have another renal abnormality, such as inadequate urinary concentration ability without another non-renal cause, proteinuria of renal cause, abnormal kidney palpation or imaging findings, or increasing creatinine concentrations in an individual patient over time.
These patients are more challenging to identify through routine examination and blood work. A knowledge of the causes of kidney disease is essential to recognising those at risk prior to the development of overt CKD.
The prevalence of CKD is estimated to be between 0.5% to 1% in dogs and 1% to 3% in cats, and increases with age.
It is estimated that up to 50% of cats older than seven years of age have CKD. CKD is progressive in both species. Early detection allows for regular monitoring and – especially – implementation of therapies proven to delay progression, prolong survival and increase quality of life of our patients.
Renal disease can be divided into congenital or acquired disease, and can present as acute or chronic disease, or acute-on-chronic disease.
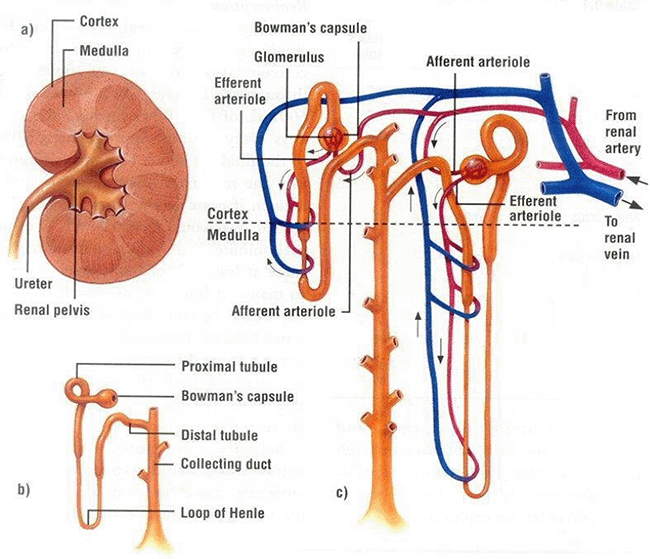
It can arise from damage to various parts of the kidney, such as the glomerulus or the tubules (Figure 2).
Congenital and familial kidney disease in the dog and cat is uncommon.
Congenital disease may arise from genetic disorder or a natural error during embryological development; they are not always inherited.
Familial diseases are present in high frequency among specific breeds; they are assumed to be inherited and causative genes have been identified in some breeds.
Congenital and familial disease are present from birth and as such are usually identified in young patients. Some conditions result in progressive and ultimately fatal renal disease, such as renal dysplasia; others are subclinical and often go undiagnosed or are incidentally found, such as unilateral renal agenesis (absence of one kidney).
The most commonly known congenital renal disease is renal dysplasia, also known as juvenile nephropathy. Renal dysplasia represents a group of development anomalies that centre around abnormal development of the renal parenchyma – most commonly affecting the nephrons.
Subsequent degenerative and inflammatory processes occur within the ultrastructure of the kidney as the disease progresses. The resulting disease takes a similar course to acquired CKD, but is usually more advanced at diagnosis than acquired CKD.
Renal dysplasia has been reported in many breeds. Most commonly affected breeds have undergone extensive characterisation (such as the shih-tzu) while other breeds are represented by small case series or a single case report, and less is known.
The most common breeds that present regularly in practice where renal dysplasia should be considered include the shih-tzu, Lhasa apso, golden retriever, cairn terrier, cocker spaniel, cavalier King Charles spaniel, bulldog and miniature schnauzer. The disease is less common in cats, with sporadic reports, such as the Norwegian forest cat.
Acquired kidney diseases include the following.
The most common acquired kidney disease in dogs and cats is CKD. It is most commonly detected incidentally on routine blood work of middle to older-aged dogs and cats in practice. Identification of azotaemia in an asymptomatic older patient is not alarming, yet it should always be further investigated to assign where possible a cause of CKD.
The majority of acquired renal disease leads to the development of CKD; however, the progression and therapy differs depending on the original diagnosis.
Glomerular versus nephronic versus tubular diseases of the kidney overlap and can be confusing.
In essence, glomerular diseases are centred around the membranes of the filtration apparatus called the Bowman’s capsule. Glomerular disease is generally renal in origin, but factors exist outside of the kidney that can damage the delicate membranes.
Examples of prerenal factors include hypertension, hyperthermia and syndromes causing hyperglobulinaemia, such as immune disease or neoplasia.
Primary glomerular diseases can be acquired or hereditary. Some examples are deposition of amyloid (amyloidosis), degenerative glomerular nephritis, immune-mediated glomerulonephritis, lymphoma or deposition of immune complex formations (either as part of the pathogenesis or secondary to prerenal systemic diseases).
The resulting damage leads to excessive amounts of protein in the ultrafiltrate and then urine (proteinuria), and eventual downstream propagation of this inflammation in the kidney structures. This damages the nephron, tubules and collecting ducts.
As only 50% of patients with glomerular disease are azotaemic, and approximately 40% can have a normal urine specific gravity (USG), it is important to pay attention to the protein content of urine of middle to older-aged patients.
Excessive protein loss through the kidney is defined as protein-losing nephropathy and leads to the development of nephrotic syndrome – a complex pathophysiological response to hypoalbuminaemia, leading to hypercholesterolaemia and oedema.
The glomerulus filters blood, resulting in production of an ultrafiltrate. This fluid passes through the nephrons, undergoing active resorption and/or excretion of solutes, followed by pH and water manipulation by the tubules and collecting ducts. The resulting urine is of appropriate volume, specific gravity, solute concentration, protein content and pH.
In a normal day of a healthy animal, less than 1% of the fluid that is filtered by the glomerulus is excreted as urine.
Tubular diseases are better known, given their rarity and unique presentations, such as Fanconi syndrome, renal glucosuria and tubular acidosis syndromes (type I and type II, affecting the distal convoluted tubule and proximal convoluted tubule respectively).
Nephrotoxins very commonly damage this part of the kidney; for example, ethylene glycol toxicity or aminoglycoside toxicity.
Acute kidney disease (AKI) is typically due to ischaemic or toxic insults, and usually affects the tubular portion of the nephron. In contrast, CKD can be caused by diseases and disorders that affect any portion of the nephron, including its blood supply and supporting interstitium.
Early detection of AKI facilitates appropriate intervention that can arrest or at least limit the cellular damage to the tubules and halt the progression through the stages of AKI. This reduces the risk of development of CKD in the recovering months.
Discerning acute from chronic or acute-on-chronic kidney disease requires a complete overview of the patient’s history, examination, blood work, ancillary diagnostics and, most commonly, time. We will discuss how to identify with reasonable certainty which scenario is present.
The identification of azotaemia should be followed by investigation into whether the azotaemia is pre-renal, renal or post-renal. This can be deduced in the majority of cases using clinical examination to assess hydration and the USG.
A physiological USG greater than 1.030 in a dog and greater than 1.035 in cat is considered to represent a prerenal azotaemia. Values lower than this indicate inadequate concentrating ability, provided the patient has not been receiving fluid therapy, does not have another disease, nor is receiving a medication that affects the action of urinary concentration by the kidney.
The diagnosis of CKD requires thorough history taking, physical examination, weight, blood pressure measurement, and assessment of blood and urine.
Serial assessment of patients in general practice on a yearly basis will often alert the practitioner of subtle signs of early kidney disease. Those considered at risk of CKD should be monitored between their annual examinations (Panel 1).
At annual health examination, we should enquire about the patient’s general appetite and water intake; often, in early CKD, no clinical signs may be present. In patients with more advanced stages of CKD, noticeable weight and muscle loss, anaemia, polydipsia, lethargy, as well as signs related to uraemia such as inappetence, nausea, vomiting and loose stools are usually present.
Patients presenting with AKI may or may not have a history of exposure to identified risk factors of AKI (Panel 2). A dog or cat with AKI is typically much more clinically unwell than a CKD patient; however, patients with AKI that only have mild clinical signs such as inappetence exist. Patients with glomerular disease and nephrotic syndrome can have oedema or ascites (approximately 15% of cases) or not (50% of cases).
The most sensitive assessment of kidney function is the evaluation of glomerular filtration rate (GFR). Measurement of GFR is costly, time consuming and invasive for our patients, hence why surrogate markers are used. Once an animal becomes azotaemic, serum creatinine correlates well with GFR, and therefore it is unnecessary to quantify GRF in an azotaemia patient for diagnosis of CKD or AKI.
However, in early kidney failure, GFR can substantially decline without any reflection on routine blood work.
In practice, GFR evaluation is indicated for selected cases, such as patients with suspect renal disease despite normal creatinine, breeds with juvenile onset kidney disease and to safely administer nephrotoxic drugs in patients with reduced kidney function.
Surrogate markers of GFR are more commonly relied on and include creatinine, urea, USG and SDMA. They are usually combined to estimate kidney GFR. Various tests become abnormal at various stages of GFR decline, and each test is subject to limitations. It is recommended to combine various estimations of GFR to stage a patient’s CKD.
As mentioned previously, patients with glomerular disease may only have proteinuria with or without nephrotic syndrome, without azotaemia or poor urinary concentrating ability.
Among some of the surrogate markers of GFR are the following.
As mentioned previously, glomerular disease can be detected with and without azotaemia. Detection of hypoalbuminaemia and hypercholesterolaemia in patients with proteinuria and UCPR values greater than two are considered to have nephrotic syndrome. In advanced stages of CKD, we begin to see changes in calcium, due to secondary renal hyperparathyroidism. In early stages of CKD, phosphorus concentrations can be normal or mildly increased.
Haematology will often detect mild non-regenerative anaemia; this is a non-specific finding and can be present in a multitude of chronic diseases – especially in cats (30% to 65%).
When accompanied by stage one or two CKD in the canine patient, it should be complemented by thyroid hormone and circulating thyroid-stimulating hormone (cTSH) assessment in patients with compatible clinical signs and examination. Anaemia is usually not detected in stage one disease.
Lack of a stress leukogram in a canine patient with AKI, with compatible clinical signs and examination, should prompt further investigation of Addison’s disease with a basal cortisol or an ACTH stimulation test. Finding an inflammatory leukogram in a patient with AKI or CKD could indicate concurrent lower or upper urinary tract infection, inflammation or obstruction. Haematology is typically normal in patients with stage one and two CKD.
Ultrasound and/or radiographic assessment of kidneys is necessary to diagnose early CKD, as well as causative or concurrent diseases in patients with CKD. Ultrasound particularly makes an excellent ancillary test for diagnosis and monitoring progression of dogs and cats with CKD. Renal contour, corticomedullary ratio and definition, cortical echogenicity, the presence of cysts, stones, mineralisation (nephrocalcinosis) or infarcts as well as pyelectasia should be assessed.
Ultrasonographic findings in dogs with CKD most commonly include loss of corticomedullary definition, abnormal ratio of corticomedullary junction and pyelectasia. The number of abnormalities in renal ultrasound in dogs increases with increasing IRIS stage.
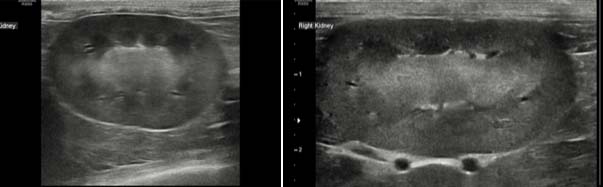
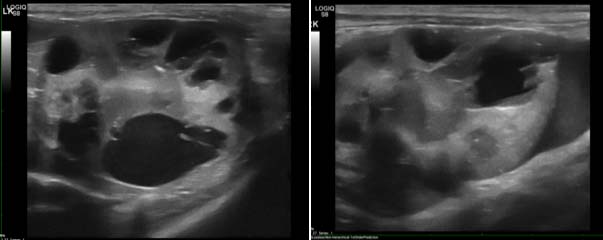
In feline patients similar criteria are noted, with poor corticomedullary definition, increased echogenicity and irregular renal contour most commonly noted (Figure 3). Polycystic kidney disease can often exist in cats without any azotaemia (Figure 4) and qualifies as stage one disease.
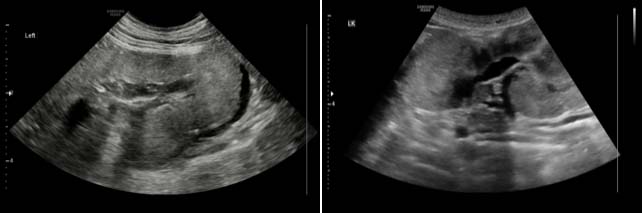
Ultrasound can identify dysplastic kidneys in young dogs with compatible history and clinicopathological changes (Figure 5). Renal architecture is typically very abnormal; small, misshapen, poorly defined kidneys bilaterally are noted.
Blood pressure measurement should be part of the assessment of geriatric healthy patients, as well as part of diagnosis, staging and management of those with documented CKD.
Patients with signs of end organ damage, such as retinal damage or left-sided ventricular hypertrophy, do not require serial measurements to prove they are hypertensive.
Hypertension is considered when systolic blood pressure is persistently 160mmHg to 179mmHg across one to two weeks and serial measurements.
Severe hypertensive is considered when systolic blood pressure is persistently greater than 180mmHg across one to two weeks of serial measurements.
Up to 65% of feline CKD patients have hypertension. A retinal examination is recommended in patients that do not tolerate blood pressure measurement, or those with documented hypertension.
Once a diagnosis of renal disease is made, and the disease has been staged as previously mentioned and according to the IRIS guidelines, a treatment plan should be developed for that patient.
The IRIS guidelines provide instruction for each stage of CKD. Generally, few extra-renal signs present at the earliest stages of CKD (stage one and two) and the therapeutic emphasis is on slowing the progression.
In later stages, management and therapy becomes more intense, as patients develop anaemia, gastrointestinal signs of uraemia, hyperphosphataemia, secondary renal hyperparathyroidism, acidosis and potassium disturbances.
Earliest recognition of renal disease requires detection of pets at risk of renal disease, physical renal abnormalities and/or urine abnormalities such as reduced USG or proteinuria in patients that are typically asymptomatic.
The mainstay method of detecting non-azotaemic CKD is awareness and annual assessment with careful history taking, clinical examination, blood and urine testing.
The most sensitive surrogate marker of early GFR decrease that is readily commercially available is SDMA, and identification of increased SDMA concentrations should be complemented with urinalysis and imaging of the kidneys to ascertain the relevance of the increased SDMA.
Patients with suspected CKD should undergo thorough staging, so appropriate monitoring and therapy can be implemented.
The goal of early intervention is to slow progression, maximise survival and optimise the quality of life of the patient.