9 Dec 2025
SGLT-2 inhibitors: what role can they play in veterinary care?
Kit Sturgess MA, VetMB, PhD, CertVR, DSAM, CertVC, FRCVS examines the operation, benefits and concerns of these drugs, which could potentially be used to manage a range of diseases.

Figure 1. Site of action of sodium-glucose cotransporter-2 inhibitors on the reabsorption of sodium and glucose in the S1 segment of the proximal tubule.
In the past three years, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, an exciting new class of drug, have become available on the veterinary market for use in the management of diabetes in cats.
Two licensed products are available worldwide: bexagliflozin and velagliflozin. Other gliflozins have been used in dogs and cats – including dapagliflozin, licogliflozin, canagliflozin, empagliflozin and sergliflozin – in clinical and non-clinical studies.
All act in a similar fashion, but they have different oral bioavailability, half-life, duration of receptor inhibition and potential tissue selectivity.
Early indications suggest that SGLT-2 inhibitors provide a very valuable treatment option for the management of diabetes in cats, and it is hoped will reduce the number of cats (approximately 10% of new cases, which equates to 10,000 to 15,000 cats per year in the UK) that are euthanised on diagnosis.
For SGLT-2 inhibitors to effectively manage a diabetic cat, the patient must have sufficient potentially functional insulin-secreting cells left in the pancreas. These cells are non-functional or poorly functional due to the effects of glucose toxicity. By lowering blood glucose through increased renal loss, glucose toxicity is reversed and the insulin-producing β islet cells in the pancreas can recover, resulting in improved glycaemic control.
As a sole therapy, SGLT-2 inhibitors are insufficient if an absolute loss of β islet cells occurs to the point that the pancreas cannot produce sufficient insulin, even if all cells were functional. This is the case in the majority of dogs and around 10% to 20% of cats. SGLT-2 inhibitors can still have a therapeutic role in these cases; a number of studies in dogs have shown that their use can reduce the amount of insulin required and improve glycaemic control (Box et al, 2024).
When SGLT-2 inhibitors were first being developed for use in human diabetics, cardiotoxicity was a significant concern. However, studies looking at this risk showed the exact opposite: SGLT-2 inhibitors had a protective effect on cardiac function. Work in diabetic and non-diabetic people has also shown that they slow or prevent progression of cardiovascular and renal disease.
One paper has shown that canagliflozin suppresses atrial remodelling in a canine atrial fibrillation model (Nishinarita et al, 2021). Use of velagliflozin in dogs or to manage non-diabetic disease in cats is off licence.
Mechanism of action
SGLT-2 receptors are primarily found in the proximal convoluted tubule of the nephron, but are also present at lower levels in the pancreas, heart and brain. They act to move both sodium and glucose from the proximal tubular filtrate back into the vasculature (Figure 1).
The glomerulus filters most of the incoming sodium, with SGLT-2 only being responsible for a small percentage of resorption, so inhibition has only a small natriuretic action, whereas it is responsible for around 90% of glucose resorption.
SGLT-1 receptors are present in the greatest density in the small intestine, but are also detected in the kidney, parotid glands, submandibular glands and the heart. SGLT-2 inhibitors have a low affinity for the SGLT-1 receptor, but some cross-reactivity is present. This cross-reactivity is why diarrhoea is the most common side effect of gliflozin medication. Self-limiting diarrhoea occurs in 50% of cats on velagliflozin during the first week of treatment, with 25% of cats experiencing diarrhoea for more than four weeks.
By competitively inhibiting SGLT-2, these agents:
- Reduce glucose reabsorption, promoting glycosuria.
- Induce a mild osmotic diuresis and natriuresis.
- Lower plasma glucose independently of insulin secretion.
- Cause a modest caloric loss.
Secondary metabolic effects include:
- Reduced glucotoxicity and lipotoxicity.
- Improved β-cell preservation (in some models).
- Altered energy substrate utilisation, increasing lipid oxidation and ketogenesis.
Clinical use in cats
From an owner perspective, a major advantage of SGLT-2 inhibitors is that they can be given orally and the dose required is not dependent on the level of glycaemic control. Velagliflozin is given as a single daily oral dose of liquid (1mg/kg), equivalent to 0.3ml for a 4.5kg cat. The dose of bexagliflozin is one 15mg tablet for cats weighing more than 3kg, given once daily.
Two large scale studies on the use of velagliflozin in cats have been reported (Behrend et al, 2024; Niessen et al, 2024) along with a small study of eight cats with hypersomatotropism and diabetes (Del Baldo, et al 2025).
Treatment success is difficult to precisely quantify, as no specific definition exists of what “success” is in the treatment of diabetic cats, and this can vary widely for different owners and cats.
However, published studies indicate non-inferiority with insulin treatment with approximately 80% of owners reporting improved quality of life within three months of treatment with velagliflozin, with many cats showing a marked reduction in blood glucose and fructosamine (Figure 2), and clinical improvement in the first seven days in a significant percentage of cats. Although hypoglycaemia (blood glucose less than 3.5mmol/L) occurs, it does not appear to be clinically significant; that is, patients showing signs of hypoglycaemia compared to hypoglycaemia that can occur with insulin treatment.
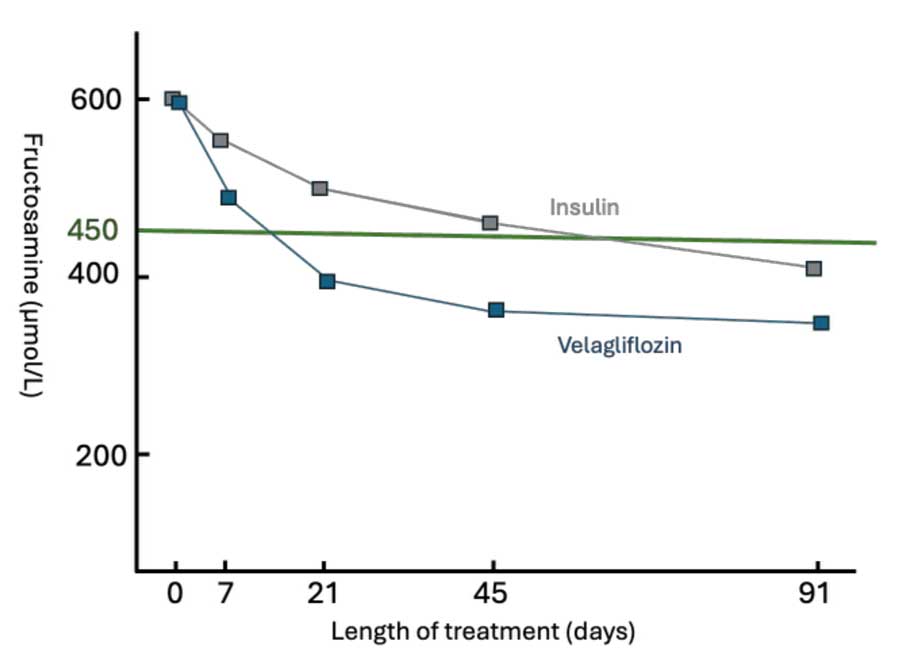
Inhibition of the SGLT-2 receptor persists beyond 24 hours, is associated with a less pronounced postprandial hyperglycaemia, and a typical responding cat will have a fairly flatline blood glucose during the day. This means that continuous glucose monitoring adds little value in assessing glycaemic control and cases can be monitored with fructosamine. It is important to realise that fructosamine assays vary widely between laboratories and some in-house tests are unreliable, so the same test system should be used to monitor a specific patient.
The major clinical concern with the use of SGLT-2 inhibitors is euglycaemic diabetic ketoacidosis (eDKA; blood glucose less than or equal to 15mmol/L), associated with the altered energy substrate utilisation – eDKA occured in around 7% of diabetic cats treated with velagliflozin, and it most commonly occurs in the first 14 days, but cases have been reported much later on in treatment.
Cats are particularly vulnerable in the early stages of treatment, as the impact of glucotoxicity on β-cell function has yet to significantly resolve; therefore, endogenous insulin production is low, so most of the energy needs of insulin-dependent cells has to be met with ketones.
Clinical presentation of eDKA is similar (depression, lethargy, vomiting, diarrhoea, inappetence, polydipsia followed by adipsia, dehydration) and treatment is also similar, but these cases are at greater risk of hypoglycaemia when given insulin, as their blood glucose is lower; therefore, dextrose-containing fluids need to be given from the start.
Cats that are already on insulin can be converted on to SGLT-2 inhibitors, but close monitoring during changeover is essential, as rates of eDKA may be higher.
Potential benefits in non-diabetic patients
SGLT-2 inhibitors are licensed for the treatment of heart failure in people in a number of countries and are associated with fewer side effects, such as hypotension and acute kidney injury.
The precise modes of action of SGLT-2 inhibitors in cardiovascular disease and renoprotection are not fully elucidated. They have a natriuretic diuretic action, which is independent of loop diuretics such as furosemide, with less activation of the renin angiotensin aldosterone system. They also cause a reduction in sympathetic nerve action and sodium hydrogen ion exchange. Whole body effects are also present, associated with the caloric loss, which impacts cell growth activation and autophagy, both important ageing mechanisms.
These various mechanisms deliver clinical benefit in cardiovascular disease by reducing blood volume and protecting against myocyte fibrosis and remodelling, oxidative stress and inflammation. Neurohormonal effects, shifting energy utilisation and changes in the cardiorenal axis may also be of importance.
In kidney disease, the main effects seem to be reduction in glomerular hyperfiltration via afferent arteriolar vasoconstriction and protection against hypoxia, oxidative stress and inflammation, all of which should lead to the preservation of functional nephrons, slowing or halting the progression of disease.
Conclusions
While SGLT-2 inhibitors are having a major impact on the treatment of feline diabetes mellitus, and may become an adjunctive treatment for some canine diabetics, they also present an exciting opportunity for managing other diseases, most notably heart and kidney disease.
- This article appeared in Vet Times (2025), Volume 55, Issue 49, Pages 6-7
Kit Sturgess graduated from the University of Cambridge in 1986, then spent six years in general veterinary practice. He has further professional qualifications in imaging, cardiology and internal medicine, as well as a PhD for looking at the effects of FIV on mucosal immune function. Kit is a fellow of the RCVS, a specialist in small animal medicine and an advanced practitioner in veterinary cardiology. He has been seeing referral small animal medicine cases for the past 25 years – both at university-based and private specialist practices. Kit’s love of teaching and learning led him to develop a new, more flexible, role combining lecturing, writing and clinic time. The majority of his clinical time is spent providing an internal medicine service at Optivet Referrals in Havant, Hampshire. Kit maintains a keen interest in many areas of internal medicine and has authored numerous articles and two textbooks, and presented lectures and research abstracts at conferences worldwide.
References
- Behrend EN, Ward CR, Chukwu V et al (2024). Velagliflozin, a once-daily, liquid, oral SGLT2 inhibitor, is effective as a stand-alone therapy for feline diabetes mellitus: the SENSATION study, J Am Vet Med Assoc 262(10): 1,343-1,353.
- Box JR, Oyama MA, Mosenco AS and Hess RS (2024). Effect of sodium-glucose cotransporter 2 inhibitor canagliflozin on interstitial glucose concentration in insulin-treated diabetic dogs, J Vet Intern Med 38(3): 1,353-1,358.
- Del Baldo F, Corsini A, Bresciani F et al (2025). Effects of velagliflozin in 8 cats with diabetes mellitus and hypersomatotropism, J Vet Intern Med 39(5): e70222.
- Elliott J and Oyama MA (2025). Sodium glucose transporter 2 inhibitors: Will these drugs benefit non-diabetic veterinary patients with cardiac and kidney diseases?, J Vet Pharmacol Ther 2025 Suppl 1(Suppl 1): 1-18.
- Niessen SJM, Kooistra HS, Forcada Y et al (2024). Efficacy and safety of once daily oral administration of sodium-glucose cotransporter-2 inhibitor velagliflozin compared with twice daily insulin injection in diabetic cats, J Vet Intern Med 38(4): 2,099-2,119.
- Nishinarita R, Niwano S, Niwano H et al (2021). Canagliflozin suppresses atrial remodeling in a canine atrial fibrillation model, J Am Heart Assoc 10(2): e017483.
