2 Jun 2021
Ian Wright takes five examples of UK zoonoses associated with cats and dogs, their current status and methods of control.

Radar image: © supawat kaydeesud/EyeEm / Adobe Stock. Worm image: ylivdesign / Adobe Stock Flea image: PMDesign / Adobe Stock
Cat and dog zoonoses represent an increasing risk to UK pets and their owners. Changes in climate and habitat in the face of increased pet travel and importation have led to significant changes in zoonotic parasite distributions across Europe, while long-standing endemic zoonoses such as toxoplasmosis remain underestimated.
To maintain the hugely beneficial human-animal bond, zoonotic risk needs to be minimised while being kept in perspective for the pet owner. This article takes five examples of UK zoonoses associated with cats and dogs, their current UK status and methods of control.
The domestication of cats and dogs has brought an increasingly close relationship between owners and their pets, with most now considered family members. They share owners’ homes, daily routines and sometimes furniture, beds and food.
Such close relationships improve physical and mental well-being, but also bring people into close proximity with zoonotic pathogens that pets may be carrying. Some cat and dog zoonoses are not directly transmitted, but their presence acts as an early warning system that people may also have been exposed.
A number of changing circumstances in the UK are leading to rises in well-established zoonotic pathogens, allowing establishment of others and increasing exposure risk. These factors include:
Despite significant zoonotic risk, some pathogens can remain under the radar of vets and pet owners because of lack of human disease surveillance or a lack of familiarity with the range of syndromes caused by the parasite. This article considers some examples of zoonoses associated with cats and dogs that appear to be increasing in incidence in the UK or are likely to be being underestimated.

The most common tick-borne pathogen of veterinary significance to UK dogs and cats is Borrelia burgdorferi, the cause of Lyme disease (Abdullah et al, 2016; Davies et al, 2017). Lyme disease also has zoonotic potential and continues to have high public awareness. Ixodes species ticks – such as Ixodes ricinus and Ixodes hexagonus, which carry Borrelia species – are endemic in the UK.
Spring and autumn peaks in activity had been demonstrated as little as a decade ago, but recent real time data from the tick surveillance scheme suggests that an extended peak now occurs from early spring though until late Autumn (Wright et al, 2018).
The reported incidence of Lyme disease in humans is increasing – 2001 saw 0.5 laboratory-confirmed cases per 100,000 people in England and Wales; this rose to 1.94 cases per 100, 000 in 2016 and 2.7 in 2017. A recent paper looking at retrospective GP records on Lyme disease diagnosis suggests these figures are a significant underestimate of the true total (Cairns et al, 2019).
The growing number of reported cases is likely to be a combination of a genuine increase in disease transmission, heightened awareness among the public and increased surveillance.
A number of factors are likely to be driving increased human exposure to ticks, with a resultant increase in Lyme disease incidence. These include increased outdoor recreational activity, increased forestation and green space, increased numbers of deer and potential reservoir hosts, and an increased seasonal duration of tick activity.
The increased numbers of housing developments in rural areas, wildlife corridors into towns and green space in urban areas produce a phenomenon known as “the crossroads effect”. This is where people and their pets are brought into closer living proximity with potential tick habitats, with subsequent tick-borne pathogen exposure. While outdoor increased activity and development of green space is to be encouraged, these increasing trends make effective tick prevention vital.
No evidence exists that dog owners might be at greater risk of Lyme disease than people without dogs, and dog ownership is not considered a risk factor for disease. Borrelia-positive dogs also pose no significant risk to owners as ticks are required for transmission. They are, however, sentinels for human infection as dog owners are likely to be at risk of tick exposure themselves from walking in the same areas if their dog has become infected.
Ixodes ricinus is also the vector for tick-borne encephalitis virus (TBEV), a flavivirus that can cause a neurological disease known as tick-borne encephalitis (TBE) in humans and, less commonly, dogs. Clinical signs include ataxia, proprioceptive deficits, seizures, tremor, paresis, paralysis and cranial nerve deficits such as facial paresis.
The European subtype of the virus has rapidly spread across central and western Europe in recent years, and maintained in endemic foci. Larger wild animals such as deer are not considered to be competent hosts for virus transmission, but serve as transport hosts for infected ticks, allowing maintenance of tick populations and geographical spread of TBEV. Migratory birds are also likely to play a significant role in carrying infected ticks over large distances.
In endemic areas, the prevalence of TBEV in questing ticks rarely exceeds 1%, even where human incidence of disease is high (Imhoff et al, 2015). The risk of human and canine infection from short visits to endemic areas with limited tick exposure is, therefore, low.
People and pets living, frequently visiting or working in endemic areas are, however, at significantly greater risk of exposure over time. This has made the possibility of TBEV establishing in the UK – in the face of an increasing European distribution, increased pet travel and pet importation – a concern as endemic domestic foci would put the UK human and canine population at greater risk. That concern now appears to be a reality, as evidence has emerged that endemic foci are present in the UK.
A surveillance programme carried out by Public Health England in 2018 looked for evidence of TBEV in wild animals and ticks. Serum was collected from 1,309 deer culled across England and Scotland; 4% of samples were ELISA-positive for TBEV with foci in the New Forest and Thetford Forest. The Thetford Forest area had the highest proportion (47.7%) of seropositive samples; a full-length genomic sequence of TBEV was also identified by PCR in one tick, confirming infection (Holding et al, 2020). This is strong evidence for TBEV being endemic in at least one endemic foci in the UK, and possibly more.
It is currently thought that these foci are small and that TBEV is not widely established in the UK, but the potential for further spread is high. I ricinus is widespread across the whole of the UK. Deer numbers in the UK are also increasing, with green corridors and increased forestation allowing easy deer movement of deer across the country and into peri-urban areas. These factors allow easy movement of infected ticks and increased exposure risk for both pets and humans.
Zoonotic risk of Lyme disease and TBE comes from exposure of people to infected tick bites. Avoiding land with high tick densities, endemic foci for TBE and highly endemic areas for B burgdorferi is the most effective way of minimising risk. These, however, are also some of the most popular and beautiful outdoor recreational destinations in the country. These areas can still be enjoyed by people employing simple preventive measures.
The European Scientific Counsel Companion Animal Parasites UK and Ireland recommends that people:
Sticking to paths where possible will greatly reduce the risk of tick exposure. Dog owners should be reassured they are at little direct risk if their pet has Lyme disease or is carrying Borrelia species infection, but they may also be exposed to infected ticks while walking their dog.
Bartonellosis (“cat scratch disease”)is a zoonotic disease caused by Bartonella species organisms. Cat fleas (Ctenocephalides felis) are carriers of Bartonella henselae, Bartonella koehlerae and Bartonella clarridgeiae, which are all zoonotic.
Transmission occurs via infected flea dirt. This can occur via abrasions from cats (“cat scratch disease”), but also via any exposure to compromised epidermis and possibly by aerosol. This is a concern as a recent study found 11.1% of flea infestations on UK cats and dogs to be positive for Bartonella species (Abdullah et al, 2019). This is higher than previously thought and likely to represent increased exposure to Bartonella in the UK population.
Ticks may also play a role in transmission. In the UK, Bartonella species were detected in 1.3% of ticks removed from cats and transtadial transmission of B henselae has been demonstrated in I ricinus ticks (Cotte et al, 2008). It is thought, however, that fleas and flea dirt are the primary source of infection.
B henselae and B clarridgeiae infections have regularly been reported in apparently healthy human hosts such as Brazilian blood donors (Pitassi et al, 2015), suggesting that asymptomatic infection occurs.
When clinical signs do occur, zoonotic infection most commonly presents as a self-limiting regional lymphadenopathy developing after a primary papular lesion lasting from a few weeks up to several months (Boulouis et al, 2005). In a minority of cases, however, this can progress to abscessation of the lymph node and systemic clinical signs, such as chronic fatigue, headaches, blurred vision and ataxia.
An increasing number of more serious atypical clinical presentations are also being recognised in association with infection, including uveitis and endocarditis (Florin et al, 2008; Tsuneoka et al, 2010). In addition, persistent infection may predispose to immune-mediated disease including anaemia, thrombocytopenia, vasculitis or glomerulonephritis as components of the disease.
The recent case of Bartonella infection being diagnosed in a boy with paediatric acute-onset neuropsychiatric syndrome, with resolution of clinical signs after treatment, demonstrates the wide range of clinical syndromes that may arise as a result of infection (Breitschwerdt et al, 2019).
Bacillary angiomatosis is one of the most common clinical presentations in immunocompromised individuals and may be fatal if untreated (Lange et al, 2009). Veterinary professionals have been identified as high‑risk groups for infection as contact with flea dirt is frequent and constant handwashing can lead to a compromised epidermis.
No first line diagnostic screening for bartonellosis exists in the UK human population presenting with many of these clinical signs and, as a result, human incidence of bartonellosis is unknown. With potential for up to 400,000 UK cats and dogs carrying Bartonella-positive flea infestations, however, this emphasises the importance of flea control to reduce zoonotic risk – especially in pets living with immune-compromised or elderly owners.
Toxoplasmosis in the UK has long been a public health concern, with enhanced human disease surveillance established in 2008. Since then, approximately 300 to 350 confirmed cases of toxoplasmosis have occurred each year in the UK, but it is acknowledged that this is likely to be a massive underestimate given the wide range of chronic syndromes the parasite has been linked to.
While healthy adults have a low risk of developing acute toxoplasmosis if infected, immunocompromised individuals or children infected in utero can suffer from severe ocular and cerebral signs that can lead to blindness or death. Chronic manifestations are also being increasingly recognised.
Zoonotic disease can be broadly grouped into three different categories.
Acquired toxoplasmosis occurs worldwide through exposure to oocysts shed in cat faeces through environmental contamination or through the consumption of tissue cysts in undercooked or raw meat. Water-borne infection, and contamination of fruit and vegetables from kitchen gardens and allotments continue to be significant sources of infection (Boothroyd, 2009).
Strains of Toxoplasma gondii in Europe and North America seem to be less virulent than those found in South America (Ajzenburg, 2012) and many cases are thought to be subclinical. Concerns about adult infection should, therefore, be kept in perspective.
Reports, though, have identified infection as a risk factor for schizophrenia, bipolar disorders, epilepsy and migraine (Flegr, 2013), with more than a fifth of schizophrenia cases potentially caused by T gondii infection. These factors and the high seroprevalence worldwide (up to one third of the world population may be infected) continue to make toxoplasmosis one of the most common and significant adult zoonoses in the developed world (Ajzenburg, 2012).
If women are infected while pregnant, transplacental infection can occur. Children infected in utero can suffer from severe, sometimes fatal toxoplasmosis. This may be local, most commonly ocular or cebral, or a generalised form affecting multiple organs.
Infections acquired during the first trimester usually result in miscarriage or abortion. Women infected during the second trimester may have children that survive birth, but with severe, sometimes life-threatening defects. Children infected in the third trimester tend to have less severe ocular or cerebral defects developing later on in life.
Infection in immunocompromised patients – such as those suffering from HIV, undergoing chemotherapy or transplant patients – can be devastating, leading to CNS, ocular or multiple organ signs.
Toxoplasmosis is a zoonosis well known by some members of the public, with some associated concern surrounding pregnancy and cat ownership. Owners should be reassured that infection poses a low zoonotic risk to immune-competent adults or to pregnant women that were seropositive prior to pregnancy. Infection, however, can carry significant disease risks and can be minimised by simple control measures.
No specific need exists for cats to be removed from households with a pregnant family member. The following precautions, however, should be advised:
Serological testing of cats is not useful as not all infected cats are seropositive and no correlation exists between seropositivity and shedding of oocysts. Consumption of undercooked meat is the most significant risk factor associated with human infection in the UK.
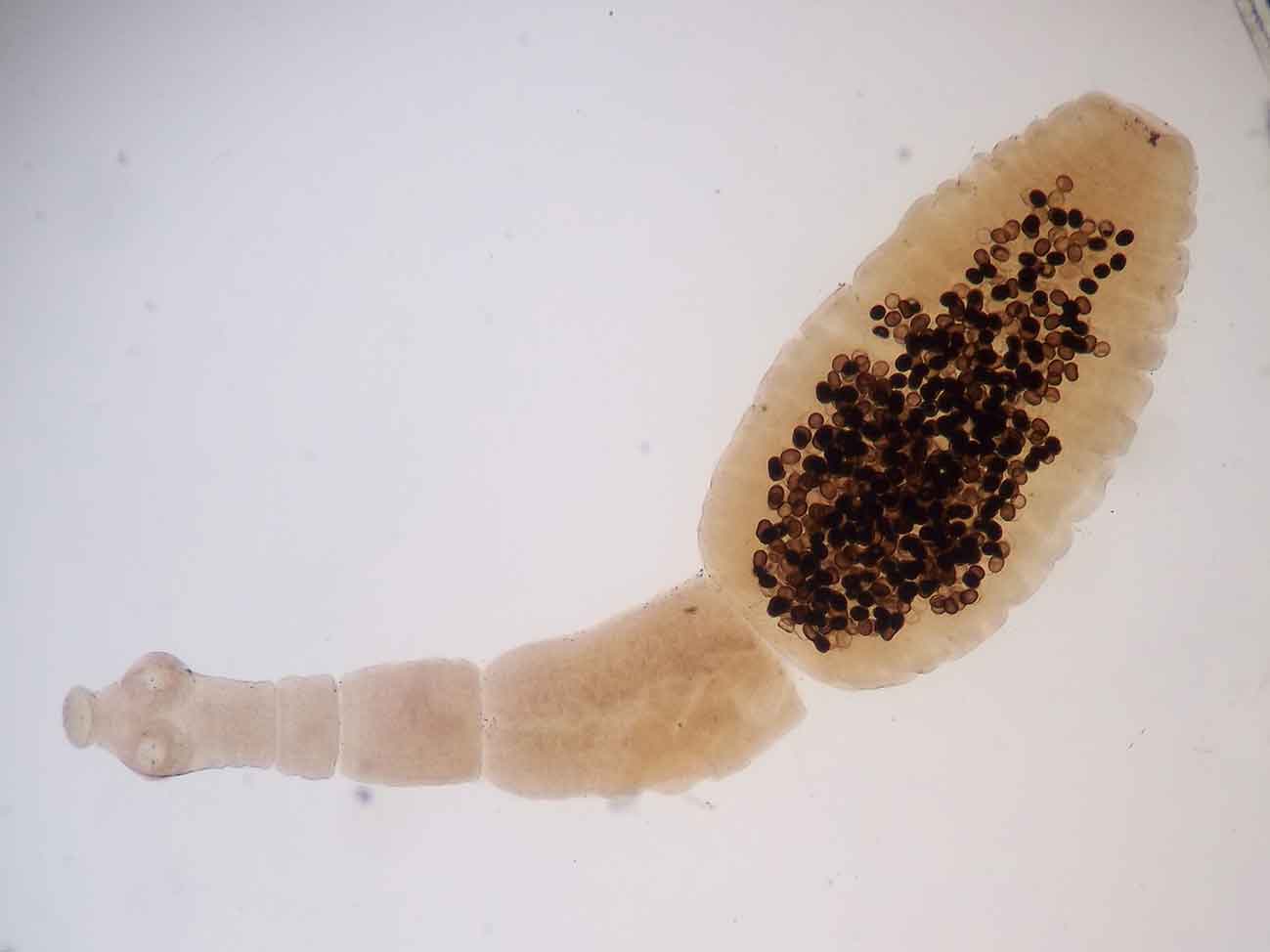
Hydatid disease has been considered historically to be limited to regional foci in Wales, the Welsh border, Herefordshire and the Western Isles of Scotland.
A voluntary control programme of supervised praziquantel dosing of dogs in Wales in the 1980s was successful in reducing the incidence of infection in dogs and ruminants, and was replaced by a health education programme in 1990. Since then, prevalence of infection in sheepdogs and livestock has reached similar levels to when control measures began.
The Welsh Assembly continues to raise public awareness of the disease and promotes praziquantel deworming programmes in dogs. Abattoir tracing work carried out on behalf of the Welsh Government found that the incidence of Echinococcus granulosus was much more widespread in England and Scotland than previously thought. Postmortem inspections in abattoirs across Britain have produced positive cases with a particularly high incidence on the Welsh border and north Midlands.
In 2015, Food Standards Agency data showed the incidence of hydatid cyst rejections in sheep and cattle offal to be 0.12% in cattle and 0.3% in sheep across England and Wales. While the possibility exists that some of these condemnations may have been misidentified Taenia hydatigena cysts, these figures still present a significant risk that dogs will be exposed to infection through offal fed directly in hunts, kennels, farms and through unprocessed diets.
Not all of these infections can be traced back to Wales, strongly indicating that endemic foci of E granulosus exist outside of Wales in dogs, presenting zoonotic risk to people in contact with them.
It is currently unknown, however, to what extent canine infection is occurring in the UK and diagnosis in dogs is difficult due to faecal flotation being an insensitive method for taeniid ova detection. Even if E granulosus ova are seen, they are indistinguishable from the eggs of Taenia species.
Coproantigen PCR tests are now commercially available, providing more sensitive faecal diagnostic techniques. Marisol Collins from the University of Liverpool has launched The HyData Project to use these tools to establish prevalence of E granulosus in hunting dogs around the UK. This data will provide valuable insight into UK dog infection and will hopefully be complete in 2021.
In the meantime, a risk-based assessment must be made to aid decisions on which dogs are at most risk of infection and require preventive treatment. This is particularly important as it can be 10 to 20 years after infection that clinical signs emerge in humans. Therefore, by the time an increase in cases of hydatid disease in humans was seen, increased exposure to the parasite would have already been occurring for a long period of time.
A number of control measures are required to reduce the zoonotic risk of E granulosus:
The zoonoses discussed are examples of a wide range of zoonotic risks associated with cats and dogs. They demonstrate the wide variety of ways that pet owners can be exposed and how sometimes exposure risks can be underestimated.
It is important that for each zoonosis, modes of transmission are considered so that transmission risks can be minimised while also keeping infection risks in perspective for clients. In doing so, pet owners can be kept as safe as possible while maintaining the precious human-animal bond.