11 Nov 2025
Top tips for recognising and managing small animal shock
Dave Beeston BVetMed(Hons), PGDip(VCP), MVetMed, DipACVECC, DipECVECC, MRCVS discusses having a structured framework to approaching patients in shock in one of a number of “My top tip” sessions at London Vet Show
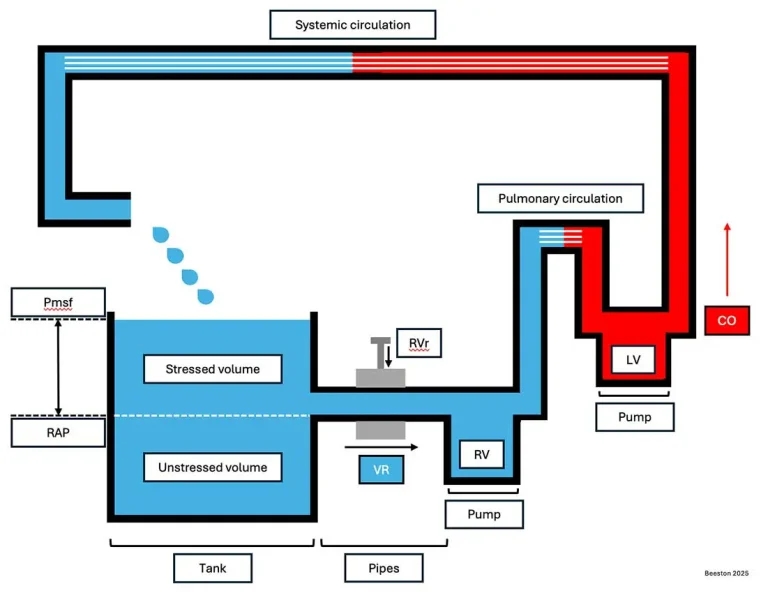
Figure 1. The tank, pipes and pump model. The cardiovascular system relies on emptying of a venous reservoir (the tank) into the heart (pump) via the venous system (pipes). See text and references for explanation of model. Pmsf = mean systemic filling pressure; RAP = right atrial pressure; VR = venous return; RVr = resistance to venous return; RV = right ventricle; LV = left ventricle; CO = cardiac output.
We have all been there: a patient gets rushed in collapsed and is poorly responsive, and a mad rush ensues trying to figure out what’s going on, all while trying to console an upset owner.
This article aims to provide a structured framework to approaching the patient in shock to streamline your case management, with some top tips sprinkled in to keep things practical.
Pathophysiology of shock
To understand how to manage “shock”, we should first understand what it is.
A thorough review of the pathophysiology of shock is available elsewhere1. In brief, shock refers to a state of inadequate cellular adenosine triphosphate (ATP) production. To produce ATP efficiently, in a process called oxidative phosphorylation, mitochondria require an energy substrate (for example, glucose) and oxygen2.
While mitochondrial failure can occur due to malutilisation of oxygen (for example, with cyanide intoxication), this is a relatively uncommon cause of shock. More commonly, failure of ATP production is due to an inadequate delivery of oxygen (DO2). The delivery of oxygen requires oxygen-carrying capacity (CaO2) and cardiac output (CO). This is commonly depicted as the “tree of life”.
CaO2 depends on the concentration of haemoglobin (an oxygen-carrying molecule), the saturation of haemoglobin and oxygen dissolved in the plasma compartment1. As haemoglobin has the largest effect on CaO2, patients with haemorrhagic shock or isovolaemic anaemia (for example, due to severe haemolysis) can have reduced DO2.
CO depends on heart rate and stroke volume, the latter of which is dependent on preload, afterload and contractility.
Tank, pipes and pump
Although the “tree of life” analogy has typically been used to describe individual shock states (for example, hypovolaemic, obstructive, cardiogenic and maldistributive), it is an oversimplification of haemodynamics and can be challenging to practically apply to our patients who rarely have a single haemodynamic problem.
Instead, a contemporary model emphasises the interaction between the venous reservoir (tank), blood vessels (pipes) and the heart (pump)3. Using the “tank, pipes and pump” model of haemodynamics can help explain the various interventions for patients in shock (Figure 1).
The venous system contains approximately 70% of total blood volume, with around 70% of this being sat in the splanchnic vasculature as a reservoir (the tank). Forward flow of blood requires a pressure gradient between the venous system and the right atrium. This reservoir can be mobilised in periods of high stress due to the effects of catecholamines on vascular tone. When the venous system vasodilates, more blood pools in the reservoir.
When the venous system vasoconstricts, more blood contributes to forward flow into the right heart. Patients with severe hypovolaemia (hypovolaemic shock) or an obstruction to venous return (obstructive shock, for example, pericardial tamponade) benefit from administration of fluid therapy to drive more blood back to the heart, although the latter necessarily requires removal of the obstruction.
Similarly, patients with reduced vascular tone (maldistributive shock, such as sepsis or anaphylaxis) will have pooling of fluid within the reservoir. Forward flow can be improved through the administration of vasopressors (for example, norepinephrine or epinephrine), as well as judicious fluid therapy.
Importantly, many veterinary patients with sepsis will also have hypovolaemia due to a reduction in fluid intake (anorexia or hypodipsia) and increases in fluid loss (third spacing into the abdomen). While these patients require restoration of circulating blood volume with fluids, early vasopressor administration may lead to reduced fluid requirements due to recruitment of splanchnic blood.
Finally, patients with poor myocardial contractility will fail to empty the venous system. Increasing right atrial pressure leads to a reduction in venous return, leading to more accumulation of fluid within the venous system. Severely impaired myocardial contractility can lead to reduced cardiac output and subsequent cardiogenic shock.
Anecdotally, pure cardiogenic shock is an uncommon emergency presentation in veterinary medicine and is typically confined to patients with dilated cardiomyopathy. However, impaired myocardial contractility may be present in other shock states, necessitating the administration of inotropes to improve cardiac output and DO2.
Perfusion parameters
The body has a fantastic capacity to deal with reduced DO2, including increasing the ability to extract oxygen that reaches mitochondria and reducing oxygen consumption.
However, reduced DO2 will lead to alterations in perfusion parameters, including heart rate, respiratory rate, pulse quality, mucous membrane colour, capillary refill time, core and peripheral temperature and mentation. These perfusion parameters act as warning signs for veterinary professionals and help identify a shocked state.
Compensation for reduced DO2 involves activation of the sympathetic nervous system and release of endogenous catecholamines (for example, norepinephrine or epinephrine). Sympathetic stimulation leads to tachycardia and vasoconstriction, typically identified by a prolonged capillary refill time, poor peripheral pulse quality (hypodynamic pulses) and cold extremities.
As vasoconstriction predominates, arterial blood pressure is often conserved or elevated – limiting the use of blood pressure as a perfusion parameter. Patients with compensated maldistributive shock will have impaired vasoconstriction leading to a vasodilated phenotype, typically identified by a rapid capillary refill time, strong pulse quality (hyperdynamic pulses) and warm extremities (Figure 2).
Veterinary professionals should pay close attention to the balance of vasoconstriction and vasodilation in their physical exam, as a vasodilated phenotype is associated with sepsis and early identification is key for patient survival.
As shock progresses and endogenous catecholamines are depleted, patients fail to compensate. Decompensation is characterised by bradycardia, vasodilation and eventually death. Importantly, cats often present in decompensated shock due to failure of initial illness recognition. Resuscitation of patients in early decompensated shock can result in recurrence of tachycardia and hypodynamic pulses, necessitating a global overview of perfusion parameters to assess improvement.
Point-of-care ultrasound
A full review of point-of-care ultrasound (POCUS) is not within the scope of this lecture. Delegates are signposted to additional training resources, including the “Techniques Lab Manual VPOCUS” document on the Calgary Veterinary POCUS Training Academy website4. Point-of-care ultrasound is invaluable in the recognition and management of undifferentiated shock.
The first priority in POCUS for shock is to rule out pericardial tamponade. Pericardial effusion can be rapidly identified by placing an ultrasound probe at the third to fifth rib space (or just behind the elbow), although in some larger patients the subxiphoid space may be required.
Pericardial effusion is characterised by an anechoic effusion surrounding the heart. Once pericardial effusion has been identified, assessment for pericardial tamponade is essential. Pericardial tamponade is most easily evaluated in the right parasternal four chamber long axis view (RPLAX-4 chamber) and can be identified by collapse of the right atrium.
Once pericardial tamponade has been ruled out, further contributors to shock, including pleural or peritoneal effusion, become the focus. Identification of free fluid should be followed by diagnostic thoracocentesis or abdominocentesis, as presence of haemorrhagic effusion may explain reduced DO2 by both a reduction in haemoglobin concentration and circulating blood volume.
Cytological evaluation of effusions for presence of intracellular bacteria is another important step, even if the effusion is grossly haemorrhagic, as patients with septic effusions require source control for stabilisation. Additional abdominal POCUS findings may include gallbladder wall oedema, which can be seen in a variety of conditions including, but not limited to, pericardial tamponade and anaphylaxis5.
Subjective assessment of cardiac chamber dilation and myocardial contractility form an integral part of cardiac POCUS. The author preferentially assesses the aforementioned in both the right parasternal short axis view at the level of the papillary muscles (mushroom view; RPSAX-papillary muscle) and the RPLAX-4 chamber view. However, neither POCUS nor formal echocardiography alone can accurately distinguish between patients with reduced preload (for instance, hypovolaemic shock) and patients with reduced afterload (such as maldistributive shock). Instead, POCUS should be combined with examination of perfusion parameters to assess the degree of vasoconstriction or vasodilation present.
A review of POCUS for assessment of intravascular volume is available elsewhere6.
Assess, intervene, re-assess
Once a shocked state has been identified using the combination of historical factors (for example, gastrointestinal losses or polyuria/polydipsia), physical examination and POCUS findings, interventions can be implemented.
Patients with suspected hypovolaemia should receive IV fluid therapy to restore circulating blood volume. In almost all situations, IV fluid therapy should be instituted with a balanced isotonic crystalloid such as Hartmann’s or lactated Ringer’s solution. A fluid bolus of either 5ml/kg to 10ml/kg or 10ml/kg to 15ml/kg delivered across 10 to 15 minutes for cats and dogs, respectively, is likely a safe start.
Veterinary professionals should move away from the concept of “shock rate fluids”, as fluids should be seen as a drug that require a specific dose and duration, as per any other drug. Following the fluid bolus, perfusion parameters should be assessed for improvement.
A lack of improvement either signifies the intervention was not large enough or fluid administration was not required to begin with. This should be correlated with the prior assessment of shock states. Repeated boluses may be necessary with some patients (for example, for severe acute haemorrhagic diarrhoea syndrome), requiring large volumes of crystalloid infusion. Infusion of blood products, including autotransfusion, may be necessary for some patients with haemorrhagic shock, although inevitably this increases cost.
Many patients with haemorrhagic shock can be stabilised with cautious crystalloid usage and prompt control of the source of haemorrhage. Patients with obstructive shock (for example, gastric dilation and volvulus [GDV] or pericardial tamponade) eventually require removal of the obstruction, although fluid therapy can help stabilise them temporarily. For GDV, fluid therapy should be administered in the cranial half of the body prior to alleviation of the obstruction, to minimise the risk of reperfusion injury.
Of note, tension pneumothorax is a cause of obstructive shock that is unlikely to benefit from fluid therapy administration, and therapeutic thoracocentesis is required to prevent imminent death.
Patients with maldistributive shock benefit from restoration of vascular tone through the infusion of catecholamines. Norepinephrine is recommended, as the first line vasopressor in human patients with sepsis7.
As norepinephrine is short acting, a continuous rate infusion (CRI) is required. An initial starting dose of 0.1mcg/kg/min is appropriate for most, with re-assessment of perfusion parameters taking place after 20 to 30 minutes. Uptitration of norepinephrine by 0.1mcg/kg/min every 30 minutes up to 0.3mcg/kg/min is reasonable, although this is case-specific and requires contextualisation.
If norepinephrine is not available, (epinephrine) can be administered, although the effects of epinephrine are less specific to vasomotor tone. In this context, epinephrine should be started conservatively as a CRI at approximately 0.05mcg/kg/min. This can be uptitrated in a similar manner to norepinephrine, although higher doses are likely to lead to tachycardia. As previously noted, pure cardiogenic shock is rare in veterinary medicine, although myocardial contractility may be impaired in other shocked states. Following assessment of volume status and vasomotor tone, patients with persistent concerns for poor myocardial function may benefit from inotropic therapy.
The author anecdotally prefers to use intravenous pimobendan (0.15mg/kg IV every eight to 12 hours) off licence for patients with subjective need for additional inotropy, although dobutamine (starting at 5mcg/kg/min in dogs) is a reasonable alternative, also used off licence, with cautious dosing required for cats (starting at 1mcg/kg/min to 2mcg/kg/min) due to reported side effects including arrhythmogenesis and seizures.
Global reassessment of perfusion parameters is required after each intervention to assess any potential benefit or detriment of the prior intervention.
Managing shock on a budget
Veterinary professionals should consider what truly constitutes a minimum database. Most patients in shock can be appropriately managed with a minimum database of packed cell volume and total solids (paying attention to serum colour), blood glucose, electrolytes, blood smear, creatinine and POCUS.
Further diagnostics are case dependent, and as with any patient should undergo a cost-benefit analysis.
- This article appeared in Vet Times Congress (London Vet Show 2025; a supplement with VT55.45), Pages 10-13 and previewed the author’s session at London Vet Show 2025.
References
- 1. Kuo K and Palmer L (2022). Pathophysiology of hemorrhagic shock, Journal of Veterinary Emergency and Critical Care 32(S1): 22-31.
- 2. Li X, Yang Y, Zhang B et al (2022). Lactate metabolism in human health and disease, Signal Transduction and Targeted Therapy 7(1): 305.
- 3. Persichini R, Lai C, Teboul J-L et al (2022). Venous return and mean systemic filling pressure: physiology and clinical applications, Critical Care 26(1): 150.
- 4. Calgary Veterinary POCUS Training Academy (2025). Empowering veterinarians with cutting-edge POCUS training, www.calgaryvpocusacademy.com
- 5. Lisciandro GR, Gambino JM and Lisciandro SC (2021). Thirteen dogs and a cat with ultrasonographically detected gallbladder wall edema associated with cardiac disease, Journal of Veterinary Internal Medicine 35(3): 1,342-1,346.
- 6. Boysen SR and Gommeren K (2021). Assessment of volume status and fluid responsiveness in small animals, Frontiers in Veterinary Science 8: 630643.
- 7. Evans L, Rhodes A, Alhazzani W et al (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021, Critical Care Medicine 49(11): e1063-e1143.
