7 Jun 2015
Update on managing feline lower urinary tract disease

Figure 7. Patient hospitalised with indwelling urinary catheter and closed collection set.
Feline lower urinary tract disease is commonly seen in practice. Patients may show chronic clinical signs or present to the clinic with acute urethral obstruction and require immediate, lifesaving medical interventions.
The RVN plays a vital role in assisting with the emergency case, but also in advising cat owners on multimodal environmental modifications, which can play a role in decreasing the recurrence.
Feline lower urinary tract disease (FLUTD) is a general term used to describe the various causes of lower urinary tract signs in cats. Common causes include urolithiasis, bacterial infection, urethral plugs or neoplasia.
Feline urological syndrome (FUS) is a term often used interchangeably with FLUTD. Feline idiopathic cystitis (FIC; also known as feline interstitial cystitis) is the most common cause of FLUTD and is diagnosed by a process of exclusion, where no specific underlying cause can be identified. FLUTD or FUS is considered one of the most common diagnoses in cats, affecting 1% annually in the UK (Gunn-Moore, 2003).
As a general rule, FLUTD is more commonly seen in:
- middle-aged cats
- neutered cats
- overweight cats
- cats that take little exercise
- cats that eat a dry diet
- cats with little or no outside access
Clinical signs
Clinical signs of FLUTD include:
- dysuria (difficult or painful urination);
- pollakiuria (increased frequency of urination);
- stranguria (straining to urinate);
- haematuria (blood in the urine);
- periuria (inappropriate urination outside of the litter box);
- vocalisation when urinating;
- prolonged attempts at urination; and
- excessively licking perineum.
Emergency cases of urethral obstruction (UO) will often display the same signs, but will be unable to pass urine. As this progresses, the patient will become more distressed. On clinical examination, the tip of the penis may be discoloured or the presence of urethral mucous plugs may be visible. Clinical signs of the “blocked cat” will progress to being depressed, dehydrated, collapse and, if untreated, ultimately death.
Management of emergency FLUTD cases
The most serious complication of FLUTD is UO. This is a true medical emergency. Common clinical signs reported by owners include stranguria, dysuria, vocalisation, lethargy, anorexia and excessive grooming of the perineum (Hall et al, 2014). On presentation, the cat will most likely have a large, firm urinary bladder that cannot be expressed, but it must be remembered the absence of a palpable bladder does not rule out UO as the patient may have had a bladder rupture.
The SAFE approach to cats with UO has been advocated (Lulich and Osborne, 2015).
- S = Stabilise first
- A = Accurate diagnosis
- F = Flush, don’t force
- E = Extend the urethra caudally
S = Stabilise first
Stabilisation is required to address the metabolic complications of UO. These complications include hypothermia, hypovolaemia, azotaemia, acidaemia, hyperkalaemia and hypocalcaemia. It was reported 12% of cats presenting for UO had electrolyte and acid-base disturbances, which is often complicated by dehydration (Drobatz, 2009).
An intravenous catheter should be placed to allow for administration of intravenous fluid therapy (IVFT). While it is in no doubt IVFT is beneficial in these cases, there has been controversy over the fluid of choice. Previously, 0.9% sodium chloride (NaCl) has been considered the fluid of choice as it does not contain any additional potassium. However, 0.9% NaCl is an acidifying crystalloid that could worsen any existing metabolic acidosis.
It is a misconception Hartmann’s solution should be avoided in cases of UO. Hartmann’s solution does contain potassium, but the benefits of dilution far outweigh this. Hartmann’s solution is an alkalinising crystalloid so helping to correct metabolic acidosis. A review of controversies in the management of feline UO stated it would appear fluid type does not have a clinically relevant impact on resolution of metabolic derangements or patient outcome (Cooper, 2015).
Blood samples should be collected for minimum data collection including PCV/total protein, blood urea nitrogen, creatinine, electrolytes and venous blood gas.
An ECG trace should be obtained as soon as practical if the patient is hyperkalaemic or the potassium has not been assessed (especially if the patient is bradycardic and/or hypothermic on clinical examination). Classic ECG changes seen with hyperkalaemia include:
- peaking and narrowing (spiked) T waves;
- short QT interval;
- widening of the QRS complex; and
- decreased amplitude or absent P waves.
Immediate treatment should be instigated for hyperkalaemia. This may include administration of calcium gluconate, glucose and regular insulin.
Analgesia
Cats with UO can be in great pain and display high levels of anxiety. There are several options for analgesia, including:
- Methadone. Methadone has a rapid onset of action (15 minutes) with a duration of effect of on average four hours.
- Buprenorphine. Buprenorphine is a potent, long acting analgesic acting at opiate receptors in the CNS. It has sedative effects after 15 minutes, with analgesic effects after 30
minutes. The peak activity is reached after 60 to 90 minutes.
NSAIDs are contraindicated in the emergency UO patient due to azotaemia and dehydration. However, they may be indicated once the patient has been stabilised.
Cystocentesis
If the bladder is moderately distended, decompressive cysto-centesis could be advantageous. Benefits include:
- Relieving discomfort and pain from a distended bladder.
- Lowering the pressure in the bladder facilitates retropulsion of urethral plugs or urethroliths, which can aid
urethral catheterisation. - Reduces biochemical consequences of UO, such as hyperkalaemia and azotaemia.
- Collection of a sterile urine sample for urinalysis and culture.
Some argue against decompressive cystocentesis due to the potential risk of bladder rupture and development of uroperitoneum. However, a study of decompressive cystocentesis in cats with UO, followed by placement of an indwelling urinary catheter, did not result in a diagnosis of bladder rupture in any cat (Hall et al, 2014).
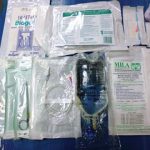
Although RVNs are not allowed to perform cystocentesis they should be aware of what is involved with the procedure including the equipment required (Figure 1). The equipment required for decompressive cystocentesis includes:
- skin prep
- appropriate size needle (1.5in, 22g)
- wide bore extension set
- three-way tap
- syringe (20mls)
- sterile sample pots
A = Accurate diagnosis
In most cases the presence of UO is a diagnosis in itself. Further abdominal imaging, including contrast radiography, may be indicated at a later stage.
F = Flush, don’t force
Once the patient is considered stable, relieving the UO can commence. Depending on the patient’s status, sedation or general anaesthesia may be required.
Sacrococcygeal (SC) epidural blocks can be used to facilitate passage of a urinary catheter in cats with urethral blockage. A study of cats that presented with UO showed successful placement of the block (performed after opioid administration +/− sedative agent) may result in easier and more rapid unobstruction and placement of a urinary catheter (O’Hearn and Wright, 2011).
Additional observations include that cats appear in less pain post-catheter placement. The study concluded using an SC block can be a valuable adjunct to the management of feline UO in the emergency room setting.
E = Extend urethra caudally
To minimise complications (urethral rupture, uroabdomen and urethral strictures) associated with passing a urinary catheter, it is very important to extend the urethra caudally and dorsally before attempting urinary catheter placement.
Prior to attempting to pass the urinary catheter, any mucous plugs may be able to be removed from the penile urethra by massaging the penis.
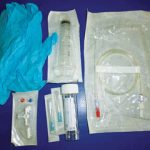
Equipment required for feline urinary catheter placement (Figure 2) includes:
- various sizes/types of feline urinary catheters (Figure 3)
- clippers
- skin prep, including a chlorhexidine flush
- sterile lubricant
- sterile gloves
- sterile saline and extension set
- suture/needle holders/scissors
- closed urine collection system (to decrease risk of nosocomial infection)
- intravenous catheter/nasolacrimal catheter (in case problems passing regular feline urinary catheter)
Technique for urethral catheterisation
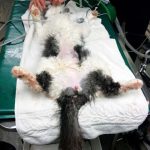
To “unblock” a cat, the sedated/anaesthetised patient should be placed in dorsal recumbency (Figure 4), then an area around the prepuce should be clipped and prepped. As previously mentioned, the urethral tip should be massaged to encourage dislodgement of any mucous plugs (any plugs should be kept for analysis).
A urinary catheter (preferably rigid and open ended) should be attached to a fluid extension set and 10ml syringe pre-filled with sterile saline. The system should be flushed to evacuate air and the catheter should be lubricated with sterile lubricant.
The urinary catheter tip should be introduced slowly into the urethral opening. As advocated by the SAFE approach, the urethra should be extended caudally (Figure 5) and the catheter then advanced slowly, until the tip lies in the bladder, while gently flushing with saline. Urine can then be removed from the bladder and the bladder can be lavaged with copious amounts of sterile fluid.
Once the urethra is cleared, a more suitable catheter to remain in-dwelling can be passed (softer and side holes).
There is controversy regarding catheter type and size – the more rigid catheters with end holes tend to help the effort to relieve the UO, but the rigidity can itself result in urethral trauma. If left in-situ they can cause more irritation compared to other materials so are not recommended as in-dwelling catheters. The softer catheters with side holes are preferred for in-dwelling use as they cause less irritation and are less likely to become blocked.
A review of controversies in the management of feline UO looked at catheter size. While it is recognised more research is needed, there is evidence from a small study to suggest a smaller (3.5Fr) urethral catheter may be associated with a decreased risk of reobstruction (Hetrick and Davidow, 2013).
Almost 50% of cats develop massive increases in their urine output following UO – a phenomenon called post-obstruction diuresis (POD; Balakrishnan and Drobatz, 2013). Increased rates of IVFT will be required to keep up with the patient’s urinary losses to prevent dehydration. The patient should be monitored for volume overload.
A closed system collection bag allows for accurate calculation of urine output (Figure 6), allowing the patients “ins and outs” to be monitored so IVFT can be tailored to suit the individual patient’s needs.
Commercial closed collection systems are available, but, alternatively, a system can be made with an empty fluid bag and fluid administration set. Patient interference with the urinary catheter should be avoided and the use of Elizabethan collars maybe indicated (Figure 7).
Another controversial area in the management of these cases is how long to leave the urinary catheter in-situ. Some argue the urinary catheter should be left in-situ for as short a time as possible, as the presence of a catheter can itself cause irritation and increase the risk of developing a urinary tract infection. Ideally, the duration of catheterisation should be determined by the clinical picture. This should factor in the presence of azotaemia, gross character of the urine and POD.
The prognosis for survival to discharge in most cats with UO is good, even in critically ill cats, providing they are stabilised within the first few hours of presentation (Balakrishnan and Drobatz, 2013).
Management of chronic cases
There is no cure for FIC. There are reported recurrence rates of 15% to 40% in cases of feline UO (Cooper, 2015), so it is vital to implement measures to reduce the chance of recurrence. Environmental stressors have been reported to exacerbate clinical signs of FIC.

In cats with severe FIC, increased concentration of circulating catecholamines have been reported compared to control cats during a period of mild stress (Westropp et al, 2006).
Multimodal environmental modifications (MEMO) therapy can be very useful and the RVN can play a vital role in the success of these therapies. MEMO therapy begins with obtaining a thorough environmental history from the owners.
Questions to ask include (but are not limited to):
- How many cats are in the household?
- Does the cat have indoor and outdoor access?
- Is there any known cat conflict?
- How many litter trays are there? (Ideally, there should be one for every cat in the household plus one)
- What litter is used?
- Where are the litter trays located?
- What food is the cat fed? (Has this recently been changed?)
- How is drinking water provided?
- Have there been any changes in the household recently?
- Are there are other signs of ill health?
Once all the information is gathered, a tailor-made plan of preventive recommendations can be devised to meet the individual needs of the owner and cat. It is recommended to make only one or two changes at a time so as to not overwhelm the owner or cat. Advice may include the following.
- Increasing water intake – perhaps the most important as only this was found to independently have an effect in decreasing recurrence (Eisenberg et al, 2013). This can be done by increasing the number of water bowls, feeding a wet diet, using fresh water fountains, using different bowl types, changing water more frequently and placing water bowls in a different location.
- Litter trays – consider more trays (perhaps with higher sides or a covered tray), changing cat litter substrate (and increasing depth of cat litter), changing location of trays and increased frequency of cleaning.
- Sleeping areas – single, cat-sized sleeping areas/perches can be provided to reduce stress in multi-cat households.
- Diet – offer a consistent diet (the same food at the same time of day).
- Playtime – ideally, cats should be provided with toys that allow them to display their natural feline predatory behaviour. Scratching posts also allow natural scratching behaviour to
be performed.
Drug therapy
The feline urethra has both smooth and striated muscle components, so drug combinations are often beneficial. Smooth muscle relaxants include acepromazine and phenoxybenzamine. Striated muscle relaxants include diazepam and dantrolene.
Nutraceuticals
Nutraceuticals are generally aimed at protecting/repairing the glycosaminoglycan layer lining the bladder. It is thought this layer becomes damages with FIC.
Pheromone therapy
It is thought pheromones induce changes in the limbic system and hypothalamus resulting in alterations in the emotional state of the animal. Cats have several pheromone-producing areas, including facial area, foot pads and perianal areas. A cat rubbing its face on its environment releases pheromones, which provides the cat with reassurance. Feliway (Ceva) is a chemical copy of one of the facial pheromones.
Conclusion
In conclusion, FLUTD is a common, but often complex, disease and the registered veterinary nurse can play a valuable role in the management of these cases, in both the acute and chronic stage.
- Please note some drugs mentioned within this article are used under the cascade.
References
- Balakrishnan A and Drobatz KJ (2013). Management of urinary tract emergencies in small animals, The Veterinary Clinics of North America. Small Animal Practice 43(4): 843-867.
- Cooper ES (2015). Controversies in the management of feline urethral obstruction, Journal of Veterinary Emergency and Critical Care 25(1): 130-137.
- Drobatz KJ (2009). Urethral obstruction in cats. In Bonagura JD and Twedt DC (eds), Kirk’s Current Veterinary Therapy XIV, WB Saunders, St Louis: 951-953.
- Eisenberg BW, Waldrop JE, Allen SE, Brisson JO, Aloisio KM and Horton NJ (2013). Evaluation of risk factors associated with recurrent obstruction in cats treated medically for urethral obstruction, Journal of the American Veterinary Medical Association 243(8): 1,140-1,146.
- Gunn-Moore D (2003). Feline lower urinary tract disease, Journal of Feline Medicine and Surgery 5(2): 133-138.
- Hall J, Hall K, Powell L L and Lulich J (2015). Outcome of male cats managed for urethral obstruction with decompressive cystocentesis and urinary catheterisation: 47 cats (2009 to 2012), Journal of Veterinary Emergency and Critical Care (San Antonio) 25(2): 256-262.
- Hetrick PF and Davidow EB (2013). Initial treatment factors associated with feline urethral obstruction recurrence rate: 192 cases (2004 to 2010), Journal of the American Veterinary Medical Association 243(4): 512-519.
- Lulich JP and Osborne C (2015). Safely Unobstructing the Urethra of Male Cats – Minnesota Urolith Centre, University of Minnesota, www.cvm.umn.edu/depts/minnesotaurolithcenter/prod/groups/cvm/@pub/@cvm/@urolith/documents/asset/cvm_asset_199031.pdf (accessed February 23, 2015).
- O’Hearn AK and Wright BD (2011). Coccygeal epidural with local anesthetic for catheterisation and pain management in the treatment of feline urethral obstruction, Journal of Veterinary Emergency and Critical Care 21(1): 50-52.
- Westropp JL, Kass PH and Buffington CA (2006). Evaluation of the effects of stress in cats with idiopathic cystitis, American Journal of Veterinary Research 67(4): 731-736.
Meet the authors
Kerry Hall
Job Title