14 Aug 2017
David Rendle and Harry Carslake discuss methods of diagnosing horses with this condition, assessing the positives and challenges of each one.
Generalised adiposity in a Shetland pony with equine metabolic syndrome.
Over the past decade, awareness of the importance of underlying endocrine disease in the development of laminitis has increased. Equine metabolic syndrome (EMS) and/or pituitary pars intermedia dysfunction (PPID) are identified in most horses with laminitis. In some cases of laminitis, a diagnosis of EMS or PPID is obvious from clinical signs, but, in others, laboratory testing can be helpful to confirm the diagnosis, assess laminitis risk and monitor treatment responses.
However, laboratory methods for assessing PPID and EMS have their limitations, as does our understanding of how we should interpret the results. Clinical research from a number of groups has resulted in advances; this article aims to summarise developments and how they can be applied in practice.
Interpretation of laboratory tests is something we do daily in veterinary practice and, for most quantitative tests, we obtain the result alongside a normal reference range. From that, the test result is normally interpreted as being within the normal range (negative for disease) or outside the normal range (positive for a disease).
However, these cut-offs are statistically derived based on populations and an assessment of probability that an individual has a disease. The boundaries become even more blurred when considering conditions such as equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID), where it is difficult to define normal versus abnormal, onset is gradual and normal physiology varies with season, diet and numerous other factors. Any cut-off imposed is, therefore, a guide and it is better to consider a spectrum of risk rather than a dichotomous classification.

The reason for testing also needs to be considered. In the context of PPID and EMS, what we actually want to know, in most cases, is whether EMS or PPID is responsible for the development of laminitis or other adverse effects of these conditions, or place the horse at increased risk of developing them. However, we often substitute reference intervals that are an estimate of whether disease is present.
When faced with a result and reference interval, we should intuitively modify this interpretation based on several test and patient factors.
Different methods in different laboratories vary in their accuracy and results can be discordant. This is particularly relevant when disagreement exists between methods used commercially and those used for research studies from which reference intervals are frequently derived. For example, population studies have generally used radioimmunoassays for measurement of insulin and adiponectin, while commercial laboratories use chemiluminescent and ELISA, respectively.
Discordance exists between methods of measuring adrenocorticotropic hormone (ACTH), probably because different methods detect different peptide sequences.
When different methods are measuring different peptides, research findings using one method obviously have to be extrapolated to other methods with caution. Fortunately, most commercial laboratories in the UK use the same chemiluminescent method to measure ACTH used in most recent research.
Precision gives an indication of repeatability and, for most methods relevant to this subject, this is fewer than 10%, which has little bearing on results. However, precision may reduce when delays in sample processing occur.
The most commonly used method for determining a normal reference interval involves sampling a group of normal horses then calculating two standard deviations from the mean value.
This results in 5% of normal horses lying outside the reference interval – 2.5% below and 2.5% above.
It is also important to consider what criteria were used to define the normal when establishing the reference range and from what population they were taken. This approach presents challenges when subclinical disease is common, as with EMS or PPID; for example, estimates have suggested 50% to 70% of the UK equine population is obese, so what is “normal”?
Cut-offs may, therefore, be based on small populations where disease status is known, such as comparisons of ponies that have had a history of laminitis versus those that have not, or studies of horses subject to euthanasia and have had histology of the pituitary gland performed. An alternative approach is to use large datasets from laboratory submissions, but this is complicated by the inaccuracy and incomplete nature of the data, uncertainty over the true disease status and inconsistency of sample handling.

Many tests have published sensitivity and specificity values, and, although useful as an assessment of the test itself, in clinical practice when a result is received, the more relevant question is: “In this individual horse, what is the chance of the result being correct?”
Positive predictive value (PPV; the probability a horse tested positive actually has the disease) and negative predictive value (NPV; the probability a horse tested negative is free from disease) are partly determined by the specificty and sensitivity of a test, but also, importantly, by the pre-test probability (PTP) of the horse having the disease.
PTP is influenced by clinical examination, results of other diagnostic tests, the background prevalence of the disease in horses of the same signalment/population and other factors depending on the individual.
For example, if ACTH was measured in a five-year-old horse with no clinical signs consistent with PPID, a positive result would be regarded with a high degree of scepticism because the PTP of the horse having PPID is very low, so the PPV will also be reduced. If we had received a negative result, we would be more likely to believe it as, with a very low PTP, the NPV tends to be much higher.
Conversely, a 25-year-old pony with hypertrichosis and weight loss has a very high PTP of having the disease; increasing the PPV, but decreasing the NPV. In both situations, the diagnostic test has not changed, but the interpretation is influenced by the individual circumstances of the case.
Subsequent discussion of reference intervals and cut-offs should be considered in the context of all of these factors.
EMS is a collection of metabolic and endocrine abnormalities associated with a high risk of laminitis and, in most cases, general or regional obesity. Previously, a diagnosis of EMS was most commonly reached in more advanced cases, following the onset of laminitis.
Increasingly, the focus is on early detection of horses with EMS at increased risk of developing laminitis, allowing preventive strategies to be implemented.
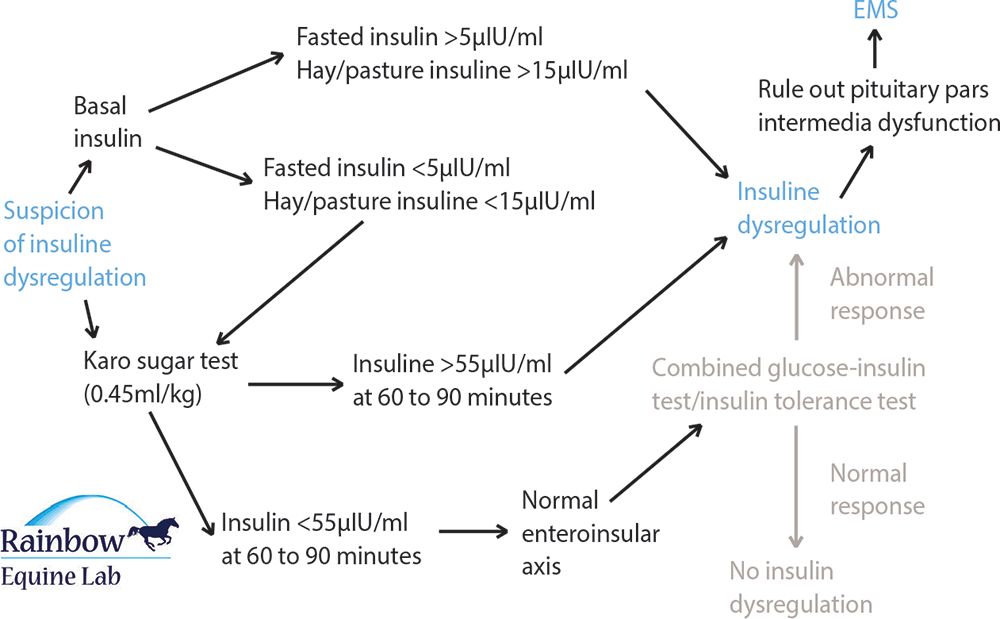
Basal insulin measurements are a convenient means of identifying insulin dysregulation and diagnosing EMS. Although their PPV is good, their NPV and sensitivity are poor; therefore, an abnormally high result is useful in diagnosing EMS, but a normal result is not helpful in ruling out the condition, so further testing is warranted.
Diet influences insulin responses and to limit effects of diet and ensure consistency, it was traditionally recommended measurement of insulin concentration be performed after a six-hour fast. However, fasting further lowers the sensitivity of measuring insulin concentration and testing is likely to be more rewarding after feeding forage that is low (below 12%) in non-structural carbohydrates. Access to grazing is best avoided, but if grazing is very restricted then testing can be performed. Feeding lush grazing or cereals within a few hours of testing will confound the results and is to be avoided.
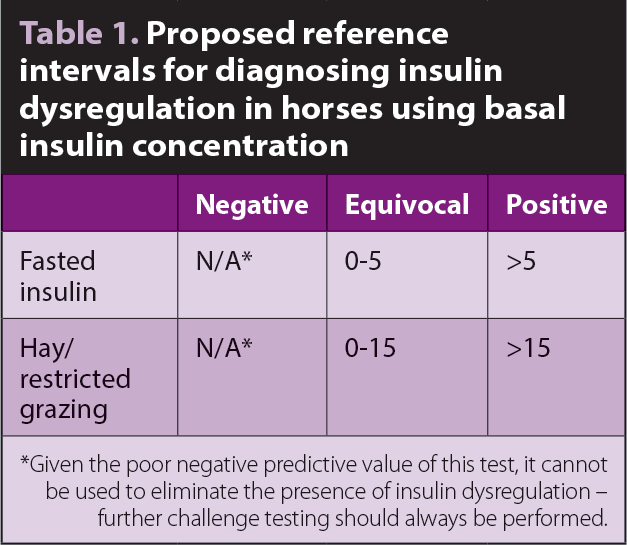
A cut-off of 20IU/ml has been used for diagnosing insulin dysregulation for many years1, but evidence has indicated this is inappropriate as sensitivity using this breakpoint is too low2. Recommended cut-offs are shown in Table 1.
If a horse suspected of having EMS has a negative or equivocal basal insulin result, further testing ought to be performed, typically using a glucose challenge test (GCT) or oral sugar test (OST).
Measurement of cytokines released from adipose tissue (adipokines), such as leptin and adiponectin, is an appealing means of diagnosing EMS.
Leptin is derived from adipocytes, and blood concentration increases proportionally with body fat deposits. Although some correlation exists with insulin resistance, leptin concentration is more reflective of the size of adipose deposits, so is not commonly used.
Adiponectin is the adipokine that has generated the most interest in the context of EMS; however, commercially available tests lack appropriate validation data and have limitations. Tests that have been used in research studies are no longer in manufacture, thus alternatives have had to be sought.
Adiponectin has insulin sensitising and anti-inflammatory functions, is secreted by fat and circulates in three main forms: trimers, hexamers or high molecular weight. High-molecular weight adiponectin accounts for the majority of circulating adiponectin, and its concentration is inversely proportional to body condition score and insulin concentration3,4.
Counter-intuitively, therefore, as adipose tissue increases, adiponectin concentration decreases. A total adiponectin concentration of below 2.5ng/ml, measured using a radioimmunoassay, has been identified as a risk factor for future laminitis5. However, results generated from some high-molecular weight adiponectin ELISA tests do not appear to correlate with total adiponectin concentration and accuracy appears to be poor. Further work is needed before adiponectin can be considered a useful marker of EMS.
In a study by Menzies-Gow et al5, basal insulin had predictive values for laminitis that were virtually the same as those for adiponectin, so until greater confidence exists in the methods used for measuring adiponectin concentration, measurement of insulin concentration is preferred. A further advantage of measuring insulin over adiponectin is insulin dysregulation and hyperinsulinaemia are implicated in the pathogenesis of laminitis, not simply obesity, so the link to laminitis risk is more direct.
Anecdotally, high-molecular weight adiponectin does not appear to change in response to weight loss using the testing methods available, which limits the value of adiponectin as a monitoring tool. Further research into appropriate commercial methods is required and different methods of measurement may need to be found, as adiponectin increased significantly in association with weight loss in one experimental study6.
Measurement of adipokines may prove to be useful in the future, but insufficient data exists to advocate their use over measurement of insulin concentration and assessment of insulin dysregulation.
1. Fast (preferably) for six hours or limit feed intake to a small amount of forage low in non-structural carbohydrates.
2. The owner feeds a mixture of:
3. The owner records the time taken for the meal to be eaten and weighs any that remains.
4. Two hours after the feed is given, blood sample for the measurement of insulin (red top) and glucose (grey top).
Several studies have evaluated the use of oral challenge tests and shown them to be a more reliable means of identifying insulin dysregulation than measurement of basal insulin concentration. However, they all have their limitations and validated reference ranges are lacking.
Some cut-offs are derived from comparisons of previously laminitic and non-laminitic ponies, which is obviously not the same as having a cut-off that identifies animals with insulin dysregulation that might ultimately lead to laminitis. Determination of appropriate cut-offs is complicated by the absence of an obvious gold standard and by insulin dysregulation being such a dynamic state in that there can be considerable variation in the same pony in a short period of time.
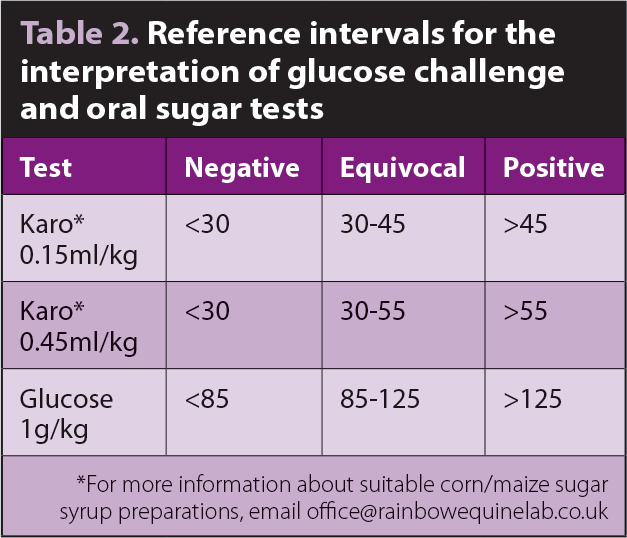
An oral challenge may take the form of powdered glucose or liquid corn/maize sugar syrup (Karo Light). Powdered glucose offers the advantage of a larger amount of sugar, hence a greater stimulus for insulin production, and the enteric reflexes associated with prehension and chewing of feed.
A disadvantage is the palatability of glucose powder, which results in the challenge being ingested slowly or partially in around 20% of cases, confounding test results (Carslake et al, personal communication). The protocol for the GCT is described in Panel 1.
1. Fast (preferably) for six hours or limit feed intake to a small amount of forage low in non-structural carbohydrates.
2. The owner administers Karo by dosing syringe – 0.15ml/kg or (preferably) 0.45ml/kg.
3. Finally, 60 to 90 minutes after dosing, blood sample twice at 15-minute intervals for measurement of insulin (red top) and glucose (grey top).
* For more information about suitable corn/maize sugar syrup preparations, email [email protected]
Karo sugar is more palatable than glucose powder and can be administered via a dosing syringe, resulting in the bolus of sugar being administered over a shorter interval, which may result in more consistent results. The protocol is outlined in Panel 2.
Work performed at the RVC has demonstrated the test is improved by increasing the dose of Karo syrup to 0.45ml/kg bodyweight9 and other authors have also recommended an increase in dose from 0.15ml/kg bodyweight10. Using a dose of 0.25ml/kg bodyweight of Karo syrup, Manfredi et al10 proposed a cut-off of 30IU/ml as evidence of insulin dysregulation using an IV test as the gold standard.
Review of data from previously laminitic and non-laminitic ponies indicated, after 0.45ml/kg bodyweight, a higher cut-off of 55IU/ml is more appropriate (Knowles, personal communication). Reference intervals for interpreting sugar tests are summarised in Table 2. Peaks in insulin concentration occur between 60 and 90 minutes after administration of Karo syrup and it is, therefore, recommended two samples are collected for measurement of insulin concentration 15 minutes apart between 60 and 90 minutes (Knowles, personal communication).
Oral challenge tests are best performed after an overnight fast, as insulin responses will be greater and more consistent. However, access to sparse pasture was not shown to alter the interpretation of the test results in one study of previously laminitic and non-laminitic ponies11.
While oral challenge tests mimic the normal physiological response to ingested feeds and stimulate the endogenous production of insulin, IV tests evaluate responses to insulin in tissues throughout the body, particularly skeletal muscle.
IV tests are more standardised, but less practical in the field. They are useful when insulin dysregulation is suspected, but is not evident following measurement of basal insulin or oral challenge tests. The IV tests favoured are the combined glucose insulin test12 and the insulin tolerance test13.
PPID is a common ageing-related endocrinopathy estimated to affect 15% to 30% of aged horses14-16. Awareness and recognition of the condition has increased considerably in recent years, prompting a shift towards earlier diagnosis and intervention. A factor often overlooked in the investigation of PPID is the presence of insulin dysregulation, which is likely to be relevant to the development of laminitis17-19.
Insulin dysregulation ought to be assessed and monitored (as described previously for EMS) in all horses with PPID. ACTH concentration is measured annually or biannually in most horses with PPID and an indication of insulin dysregulation would be equally valuable at these times – particularly in horses that have a history, or are perceived to be at risk, of laminitis.
1. Collect blood into ethylenediamine tetra-acetic acid tube (purple top).
2. Chill the sample as soon as possible (ideally within three hours).
3. Separate the plasma via centrifuging or gravity.
4. Freeze the plasma if centrifuged or refrigerate if gravity separated.
5. Ship to the laboratory for analysis within 24 hours or a maximum of 48 hours. Samples can be frozen for weeks.
Basal ACTH is accepted as a reliable screening test for PPID (Panel 3). Estimates of sensitivity and specificity vary between studies and with different inclusion criteria.
When comparisons are made between horses with advanced histological changes and horses with no histological change, the test performs very well, with sensitivities and specificities in excess of 80% and 90%, respectively20-22.
In clinical practice, the challenges for this diagnostic test are greater as the test is typically applied to cases that may have early disease, rather than being used in cases that are clearly healthy or diseased. Large numbers of borderline results therefore occur, with some false positive and false negative results being inevitable.
When the test is applied to a population with a lower incidence of disease (for example, it is used speculatively as a screening test or in horses with vague clinical signs, rather than for confirmation of disease in horses with clinical signs), the PPV of the test drops and the number of false positive diagnoses increases. Recommendations are for horses within a “grey zone” to be subject to further testing (Table 3).
A proportion of horses with low positive ACTH results that might otherwise have been treated will subsequently test negative on a thyrotropin-releasing hormone (TRH) stimulation test (TRHST), so further testing of these cases is important. The opposite is also true – some horses with PPID will have a normal basal ACTH concentration, but will have an abnormal response to TRH, given the greater sensitivity of the TRHST.
In clinical practice, multiple factors can influence ACTH concentration and, potentially, confound results. Most of these factors can be controlled and most have a limited effect on ACTH concentration, but, in isolated cases and horses with borderline ACTH levels, these confounders may be sufficient to generate an erroneous diagnosis. Plasma ACTH concentration can vary markedly in some horses – particularly those with PPID – leading to marked fluctuations in ACTH concentration within the same animal over short periods of time23, which can be an issue when serially monitoring responses to treatment.
The use of TRH to maximally stimulate the pituitary gland is likely to reduce the relative importance of potentially confounding factors, thereby increasing accuracy.
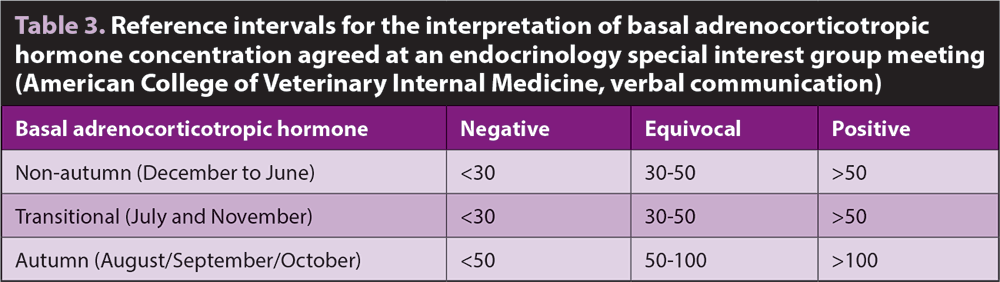
Season, and specifically day length, has a major influence on pituitary activity and, hence, ACTH concentration. Reference intervals must be adjusted accordingly. Different groups and laboratories have historically used different cut-offs, with studies from the US having advocated the use of higher cut-offs than typically used in the UK24,25. However, a consensus was achieved and summarised in Table 3 (Hal Schott, personal communication).
Differences in reference intervals between laboratories are of little concern when it is accepted a wide grey zone exists around any cut-off. As outlined at the start of this article, it is impossible to impose a dichotomous classification on a disease that is highly variable and progressive in nature.
Any ACTH value should be considered as an indication of the horse likely having PPID, and alongside clinical findings and signalment. This is not always easy when horse owners expect black or white answers.
1. Avoid concentrate feeding for three to four hours prior to testing (preferably).
2. Collect blood sample – ethylenediamine tetra-acetic acid (EDTA) plasma – for basal adrenocorticotropic hormone measurement.
3. Inject 1mg TRH IV (regardless of horse size).
4. Exactly 10 minutes after administering the TRH, collect a second blood sample (EDTA plasma).
5. Handle samples as outlined in Table 3.
The TRHST is considered to be the most reliable means of diagnosing PPID. The concentration of ACTH is measured 10 minutes after injection of 1mg IV of TRH to maximally stimulate production from the pars intermedia (Panel 4).
The TRHST should be used for any grey zone cases. It is, however, inexpensive, quick and safe, and no reason exists as to why it cannot be used as a first line test for PPID.
In cases that are younger or have less convincing clinical signs, it is worth using the TRHST from the outset to increase diagnostic accuracy and avoid the expense and inconvenience of having to perform a second round of testing in the event of an equivocal basal ACTH result.
Prior to administering, TRH owners should be warned the horse may cough, yawn, exhibit flehmen, chew or have muscle fasciculation immediately following administration; however, these signs are short lived. Serious side effects of TRH administration have not been reported.
The TRHST used to be difficult to perform because of the expense and poor availability of TRH. However, an investigation demonstrated TRH is stable and efficacious after freezing and thawing, and remains stable and efficacious for at least 18 days, if stored at room temperature26. This makes it possible for TRH to be reconstituted and frozen in the practice then removed from the freezer within 18 days of administration. Although TRH will remain stable, sterility should also be considered when vials are being stored at room temperature, so it is best kept frozen.
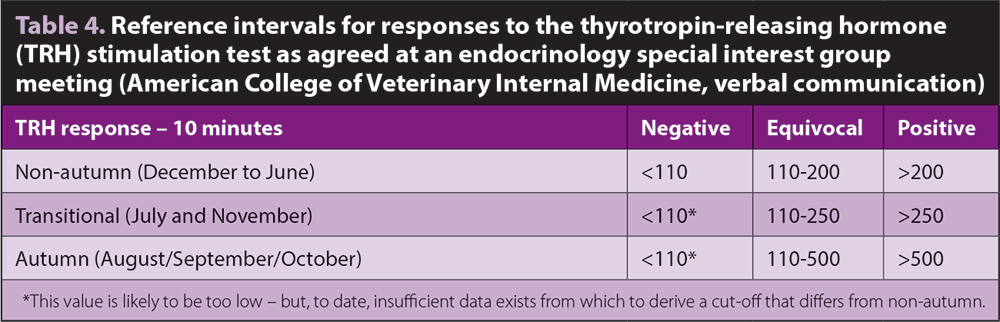
Seasonal variations in TRH responses have been characterised24, enabling the test to be used year-round (Panel 4; Table 4). Although data is limited, the TRHST can be used to assess the response to treatment with pergolide.
Insulin dysregulation ought to be assessed and monitored (as described for EMS) in all horses with PPID to assess laminitis risk and enable feeding to be tailored. Insulin dynamics also have a major bearing on how horses with PPID should be fed.
Performing an OST does not affect subsequent basal ACTH concentration, so these two tests can be combined and samples collected for ACTH, insulin and glucose 60 minutes to 90 minutes after the administration of liquid corn/maize sugar syrup27. Performing an OST was shown to reduce the subsequent ACTH response to TRH by a mean of 25pg/ml in one study27.
In the context of the inherent variability of responses to TRH, this does not necessarily preclude performing a TRHST in conjunction with an OST; however, if possible, it would be better to perform the tests on different days.
In practice, this is unlikely to happen, so, on balance, it is likely to be better to have the information provided by the OST and accept the response to TRH may be slightly diminished.
Testing for any endocrine disease in any species presents challenges, and this is particularly true of the endocrine diseases that may predispose horses to laminitis, as our understanding of these conditions is limited.
Great progress has been made to improve our ability to diagnose EMS and PPID, and assess laminitis risk. However, the tools we have at our disposal remain crude and test results have to be interpreted with caution.
Dynamic tests, such as the OST and TRHST, offer considerable advantages over basal tests and, as they are safe, straightforward and inexpensive, they should be considered as first line tests. The value of assessing insulin dysregulation in horses with PPID should not be overlooked and should be considered routine, alongside measurement of ACTH concentration.