7 Jan 2020
Fleur Whitlock BVetMed (Hons), MRCVS and Richard Newton BVSc, MSc, PhD, DLSHTM, DipECVPH, FRCVS discuss how clinicians can optimise their use of licensed medications.
Image: romul014 / Adobe Stock
With multiple vaccines on the market for a plethora of equine infectious diseases, it can be tricky to know how to guide clients to vaccinate and what populations to use each vaccine in and when.
This article will provide an overview of the available licensed vaccines in the UK and assist in guiding clinicians to optimise their use of these vaccines.
Vaccines provide a complementary measure to control infectious diseases, but their effectiveness will be determined by multiple factors and their use alone should not be relied on for complete disease control.
Reminding owners of this fact at the time of vaccination is essential to ensure they understand and adopt other precautionary measures as necessary.
It is also beneficial for clinicians to familiarise themselves with the epidemiology of each infectious disease discussed, as successful approaches will only be achieved when clinicians have a clear understanding of how each infection presents, spreads in populations, and is diagnosed, controlled and prevented.
You’d be hard-pressed to have not seen the coverage equine influenza received during 2019.
As an endemic disease in the UK, infection levels will vary year by year – with 2019 seeing an unprecedented number of laboratory confirmed outbreaks.
Virological and epidemiological factors for outbreaks are always investigated by the AHT, by using information and samples obtained from vets through the Horserace Betting Levy Board (HBLB)-funded equine influenza surveillance scheme, Equiflunet.
The reasons for the increase in the number of outbreaks reported in 2019 are probably numerous and may include factors specific to the UK equine population. These include a lack of application of preventive biosecurity measures, particularly to new arrivals, with animals arriving on premises believed to have been responsible for causing more than a third of UK outbreaks.
The UK also has a high level of horse movement and mixing, and a presumed low level of national herd vaccination coverage to equine influenza, which should, ideally, be improved and maintained. Approximate best estimates are that only between 30% and 40% of the UK horse population are vaccinated against equine influenza, so the situation seen this year should not come as a surprise.
Virological evaluation of the 2019 influenza virus confirmed the Florida clade 1 strain of the H3N8 subtype was involved.
Further in-depth analysis looks to compare identified strains with those previously found circulating and with the strains in vaccinations.
About 11% (25/227) of outbreak reports in 2019 involved vaccinated horses being confirmed positive for equine influenza. Lay interpretation of this figure may conclude vaccines had been ineffective, but this wouldn’t be an accurate assessment. It has long been known influenza vaccination will not provide 100% protection in any species, but is licensed in equids to reduce clinical sign severity, duration and viral shedding.
The level of protection obtained from vaccination will vary between horses and research has demonstrated some horses respond better to vaccination than others. Studies have also shown an improved level of immunity is obtained in horses that have received a greater number of vaccine doses in their lifetime (Barquero et al, 2007; Ryan et al, 2015).
Time since last vaccination will also play a part, with a greater level of protection in those that have received a vaccine more recently (Newton et al, 2000).
The population in which the horse resides must also be remembered – it is no good only vaccinating a subset of horses and hoping those vaccinated will have protection in the face of a high level of viral shedding by unvaccinated in-contacts.
The success of vaccination relies on high levels of population vaccine coverage – and the closer we can get populations to 100% vaccinated, the better.
Influenza virus relies on host-to-host chains of transmission to persist in the population, is incredibly contagious between fully susceptible animals and has been shown to spread over extended distances of hundreds of metres in certain optimal weather conditions. Vaccination works to break these chains of transmission and stop the onward spread of the virus.
Available vaccines must be regularly assessed to ensure they are providing protection to the circulating influenza strains. The strains to be included in vaccines are discussed annually on behalf of the World Organisation for Animal Health (OIE) by an expert surveillance panel (ESP) made up of world-renowned specialists in influenza.
The ESP collates circulating strain information from throughout the world – and decisions to update vaccine strains are determined by analysis of the existing strains, and how they are infecting and spreading in different populations.
Perhaps we were lucky in 2019 that our available vaccines provided a certain level of protection to the circulating strain in those horse populations where vaccines were used optimally.
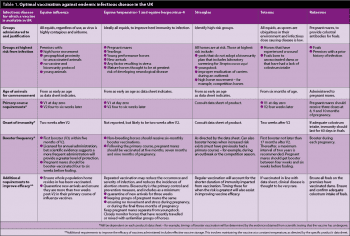
Table 1 demonstrates what the optimal use of influenza vaccination involves. However, given the changing nature of influenza viruses, we must continue to sample suspect clinical cases, and closely monitor the epidemiological and virological information.
The future effectiveness of equine influenza vaccination relies on this information and on manufacturers updating vaccines in line with it in as timely a manner as possible.
A horse’s first encounter with equine herpesvirus (EHV) commonly occurs as a foal, with the subsequent potential that the virus becomes latent post-infection. In the future, the possibility exists for reactivation, such as at times of stress.
Disease presentations can range from subclinical infections, mild respiratory signs, single cases of abortion and/or mild neurological deficits, to large groups of mares aborting or severe debilitating neurological disease that requires horses to be euthanised.
Key management practices can assist in reducing the possibility of outbreaks and lessening outcome severity if cases occur.
Vaccination against EHV is commonly undertaken in pregnant mares, but could the equine population benefit again from a population approach to infection prevention and control?
The available vaccine in the UK has a licence to reduce clinical signs due to infection with EHV-1 and EHV-4, and to reduce abortion caused by EHV-1 infection. Given this, vaccination should be adopted alongside other measures – such as keeping youngstock and pregnant mares completely separate, quarantining new arrivals for three weeks, and heightening clinical monitoring of horses at times of increased risk, such as during the competition season.
Vaccinating pregnant mares is deemed one of the cornerstones of control of abortion adopted by the industry and is promoted through the HBLB’s Code of Practice. Furthermore, the vaccine has been specifically licensed for abortion, having presented appropriate data to the licensing authorities for this syndrome.
Existing EHV vaccines do not have a claim against the neurological form of EHV-1 and a scientific paper from the US demonstrated vaccination against EHV within the preceding five weeks of infection was among several statistically significant risk factors for developing neurological EHV-1 (Traub-Dargatz et al, 2013).
Therefore, in light of this finding, vaccination of in-contacts that may have been exposed to EHV-1 during an outbreak is not usually recommended by the AHT; it will also interfere with screening and clearance diagnostic testing.
Vaccine research is ongoing, and the AHT has recently commenced a project that hopes to develop a new and improved vaccine.
Streptococcus equi continues to be responsible for many outbreaks of strangles in the UK.
With the potential of a carrier state and clinical presentations commonly being milder (sometimes referred to as “atypical”) due to a high probability of prior exposure, effective prevention and control of strangles is only possible with a good understanding of the disease’s epidemiology. Using the available diagnostic tests efficiently and correctly will also help achieve prevention and control.
Given the ever-present risk of inadvertent exposure from a “healthy carrier”, vaccination can assist to reduce the number of horses that develop the disease if they are exposed to the bacteria. However, duration of immunity is short-lasting and horses must, therefore, be booster-vaccinated frequently.
The available licensed vaccine is based on a live attenuated strain of S equi administered submucosally in the inside of the upper lip. The vaccine does not have the capability for differentiation between infected and vaccinated animals (DIVA capability), which can have wider implications if pre-movement screening or serological testing during an outbreak is required. This is because the vaccine will, itself, trip positive agent detection and antibody tests.
Therefore, use of this vaccine will generally preclude the possibility of establishing that S equi has been eradicated from a population it continues to be used in.
Recent research advances have enabled the genome of S equi to be sequenced, providing new opportunities for vaccine development – a vaccine comprising eight S equi proteins was found to provide a good level of immunity in vaccinated animals when challenged with infection (Robinson et al, 2018).
Another benefit of this new protein-based vaccine is that it has DIVA capability. However, as with other bacterial directed vaccines, duration of immunity is still not optimal – but using the vaccine in an optimal way to account for this fact will be incredibly important to enable its success.
This will involve booster-vaccinating previously primed horses at times of increased risk, such as during an outbreak or ahead of the competition season, when moving and mixing of horses – and risk of exposure to S equi – increases.
Horses are very susceptible to tetanus as a relatively small amount of potent toxin can be a lethal dose. A horse’s environment is also high-risk, as Clostridium tetani organisms are ubiquitous and spores can survive for many years in the soil.
Risk factors for infection include:
Although not contagious between horses, spores pose a risk to all horses and disease is often fatal.
Tetanus toxoid (inactivated toxin vaccine) should be administered to all horses, and provides reasonably quick and almost complete protection when administered at intervals recommended in the product data sheet.
Mares should be booster-vaccinated late in pregnancy to provide maternally derived antibody for foals and protection to the mare at the time of foaling. Vaccines should be commenced in foals as early as the data sheet allows.
If unvaccinated horses sustain injuries, they should be vaccinated and be given tetanus antitoxin alongside, administered at different injection sites.
Rotavirus is a potential major cause of infectious diarrhoea in foals. Measures are advisable to reduce morbidity and potential mortality from this disease.
Vaccinating mares – and ensuring adequate colostrum intake of foals – has been shown to provide foals with protective antibodies and reduce outbreak incidence.
Equine viral arteritis (EVA) can affect all equids and is a notifiable disease in the UK.
Prior to the confirmation of three outbreaks in the UK in 2019, the last reported case in the UK was in 2012.
Being endemic in many parts of mainland Europe, the majority of cases being subclinical and the potential for a chronic carrier state giving rise to viral shedding in semen in stallions; post-import testing, heightened vigilance and pre-breeding screening are more important than ever for prevention of this disease.
A key prevention measure includes vaccination of breeding stallions – this is promoted through the HBLB’s Code of Practice. Stallions must be confirmed to be seronegative prior to initiating vaccination, as the available vaccine does not have DIVA capability.
During 2019, cases of West Nile virus (WNV) – a mosquito-borne flavivirus – were reported in numerous European countries, including Germany, where the disease first occurred in 2018 and is the most northerly WNV has been diagnosed in Europe.
WNV is a notifiable disease in the UK. Infection can result in approximately 33% mortality in horses demonstrating clinical signs – and horses that survive may have lasting complications.
Several vaccines are available in Europe and licensed to reduce viraemia, and the severity and duration of clinical signs. Vaccination may be recommended for horses in the UK that travel to regions with endemic WNV during the vector season.
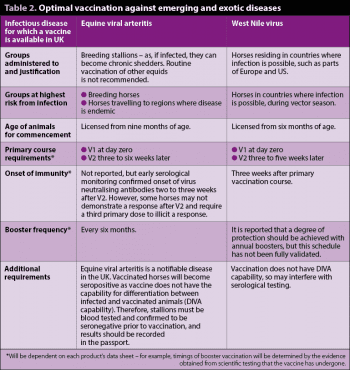
Optimal vaccination against emerging and exotic diseases is detailed in Table 2.
As demonstrated in this article, no one correct use of vaccines exists. The key overriding message is to ensure a broad awareness of the epidemiology of each infectious disease to apply preventive measures – such as, but not only, vaccination – in the most optimal ways.
Emerging and exotic diseases should also remain on our radar, and further research is required to assure our preparedness for such eventualities.
Ongoing vaccine research in endemic, emerging and exotic diseases is paramount to continue to ensure, complement and improve our defences against these ever-present threats.