1 Mar 2021
Kate Loomes BVSc(Hons), MSc, CertAVP(EP), CertAVP(VA), CertAVP(EM), DipECVAA, MRCVS in the second of a two-part article, looks at the issues that can arise before and after horses are under anaesthesia.
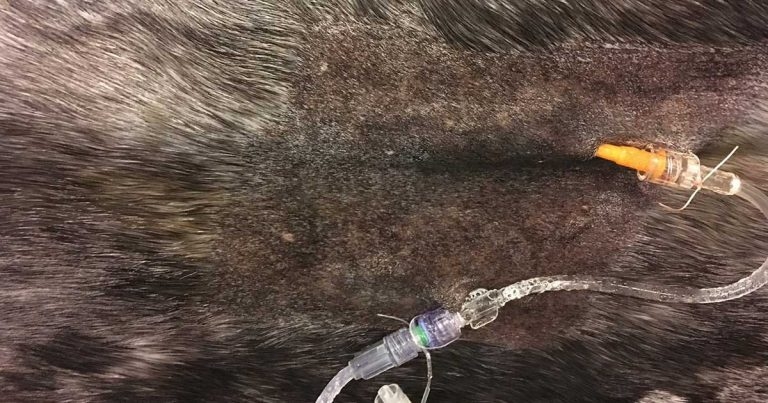
Figure 1. An IV cannula secured using sutures and connected to a short extension set.
Anaesthetic-related complications can occur at any time during the preparation of the horse for anaesthesia through to the recovery time and early postoperative period.
Continuous vigilance and close observation of response to interventions can help to prevent error, enable early detection of an adverse event and allow treatment options to be implemented where necessary.
However, the physical size of horses, their unpredictable temperament and physiological considerations all contribute to anaesthetic‑related morbidity and mortality.
The placement of a venous cannula is recommended in all horses undergoing general anaesthesia for ease of venous access and administration of fluids or emergency drugs where necessary.
Aspiration of blood via the cannula to confirm patency and correct placement is recommended prior to injection of any drug. Lavage with heparinised saline should be performed between drugs being administered and after the final drug administered to prevent inadvertent mixing of drugs within the cannula lumen, which can result in precipitation. Securing the cannula with sutures may help to prevent dislodgement (Figure 1).
IV administration of tranquillisers or sedatives may be required to enable placement of a venous cannula in some horses and inadvertent intra-arterial or perivascular injection may occur – particularly if the horse is fractious or difficult to handle.

Inadvertent intra-arterial injection is usually immediately apparent, and the severity of the neurological response may be dependent on the specific drug and dose administered. Intra-arterial administration of sedatives causes muscle rigidity, recumbency, paddling and convulsions. The therapy is symptomatic and includes muscle relaxants, such as diazepam or midazolam, to control seizures and self-trauma.
In the event of intra-arterial administration of drugs during the preparatory phase of general anaesthesia, the procedure should be postponed until complete recovery of any signs occurs.
Perivascular drug administration may initially only be suspected due to the lack of expected drug response. Depending on the pharmacological specifics of the drug, perivascular drug administration can cause focal tissue irritation, resulting in focal pain and tissue inflammation.
If suspicion of perivascular administration exists then large volumes of saline (500ml to 1,000ml) can be infused into the area to dilute the drug and the application of a hot pack can help to reduce inflammation. Perivascular administration of agents such as thiopentone or phenylbutazone can be particularly irritant and cause extensive tissue sloughing.
I
n the human medical literature, extensive research into medication error exists. A chain of events may exist where a series of minor events combine by chance and result in an accident. This chain of error causation may involve the prescriber, the person administering the drug, a lack of communication, the environment, the drug formulation, the presentation of the drug or the patient (Wheeler and Wheeler, 2005).
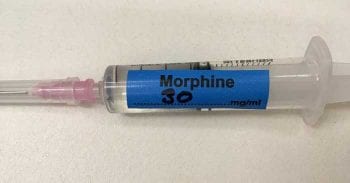
Medication error exists in all areas of human and veterinary medicine, but the fact some drugs are intrinsically more harmful than others means that medication errors in anaesthesia may be more likely to be detected and reported. In veterinary anaesthesia, it is good practice to ensure that drug syringes are labelled (Figures 2 and 3).
In the event of medication error, the action taken very much depends on the nature of the error, or the magnitude of the overdose or underdose. Inadvertent detomidine overdose has been successfully antagonised using IV and IM atipamezole (Concetto et al, 2007).
Acepromazine can cause “fainting” in excited or hypovolaemic horses. Excited horses are likely to have high levels of circulating catecholamines and the resulting beta‑2 adrenoreceptor-mediated vasodilation becomes potentiated by the antagonistic effects of acepromazine at alpha‑1 adrenoreceptors.
In hypovolaemic horses, a compensatory increase in sympathetic tone results in peripheral vasoconstriction and centralisation of the remaining blood volume.
Administration of acepromazine causes peripheral vasodilation, which can act to destabilise the compensatory response, and can cause hypotension and syncope. Profound hypotension and inadequate cerebral perfusion can result in ataxia, collapse and unconsciousness.
IV crystalloid fluid administration (10ml/kg-1 IV) and vasopressor therapy, such as phenylephrine administration, may help to improve the degree of vasodilation via agonist effects at alpha‑1 adrenoreceptors (Dugdale, 2010).
Allergic or hypersensitivity reactions are relatively uncommon in anaesthetised horses and when they occur, a spectrum of clinical manifestations may be seen, including urticaria, mucosal oedema, airway oedema, bronchoconstriction, hypotension or cardiovascular collapse. The inciting cause may not be known, but if local or systemic reaction is noted during administration of a substance then further administration should be ceased immediately.
Treatment depends on the nature and severity of the reaction, and moderate reactions often respond to corticosteroid (dexamethasone 0.02mg/kg-1 to 0.04mg/kg-1 IV) or antihistamine administration.
Blood pressure should be closely monitored during a suspected allergic reaction and therapy to support the cardiovascular system, such as vasopressors and IV fluids, may be required. In acute severe crisis, epinephrine may be required.
Manual restraint using squeeze gates or personnel may be practised, keeping the horse as still as possible against a padded wall, during the induction of general anaesthesia. “Free fall” inductions are also practised where the horse may be held in the centre of the room and the head is steadied as induction of general anaesthesia occurs.
The nature of the transition to recumbency can be variable depending on the induction agents used, the adequacy of sedation and muscle relaxation achieved prior to general anaesthetic induction, and the temperament of the individual horse.
Injury can occur to the horse or personnel during the induction phase and careful consideration should be given to safety of those involved when selecting the proposed induction technique. Producing adequate sedation may be the single most important factor determining safe and uneventful induction of general anaesthesia in horses (Muir and Hubbell, 2009).
Reasons for an inadequate response to IV anaesthetic agents at the time of intended induction of general anaesthesia may include:
Measures taken to avoid these complications include:
Absolute avoidance of injury at induction can be difficult to totally prevent in horses, given their large size and the time frame within which induction of general anaesthesia occurs. Subluxation of the elbow joint has been described in a pony that was suspected to have occurred at induction of general anaesthesia despite assisted induction with experienced handlers (Senior et al, 2002).
Intubation of the trachea in horses is generally done blind and is usually straightforward. If intubation difficulties are encountered, endoscopic visualisation and guidance can be useful. Upper airway obstruction, such as bilateral laryngeal paralysis, can obstruct the advancement of an endotracheal tube and a smaller diameter tube may be required, or tracheostomy tube placement.
Donkeys have a more extensive pharyngeal recess compared to horses and the entrance to the larynx has a more caudal angulation with a narrowing of its dorsoventral diameter (Matthews and van Loon, 2013). These anatomical differences can complicate endotracheal intubation and necessitate endoscopic guidance.
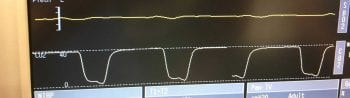
The anaesthetic machine and associated parts, such as a mechanical ventilator, should be leak tested prior to use each time. One-way valves can malfunction, allowing the rebreathing of expired carbon dioxide. This is usually reflected by a capnograph trace that fails to return to baseline and an increasing inspired concentration of carbon dioxide (Figure 4).

Large leaks in the breathing system can lead to inadequate anaesthetic agent concentration in the inspired gases, resulting in environmental contamination and an unexpectedly light plane of anaesthesia. If mechanical bellows are in use, the bellows may fail to completely fill at the end of each cycled breath if a system leak exists (Figure 5). Exhausted soda lime or carbon dioxide absorbent will result in rebreathing of expired carbon dioxide (Figure 6).
The development of post-anaesthetic myopathy/neuropathy has been associated with several factors, including:
Often no signs are apparent during the intraoperative phase of general anaesthesia that a myopathy or neuropathy may be developing and the first signs of neuromuscular injury may only be apparent in the recovery phase when the horse attempts to stand without success.
Protraction of the limb is often lacking and an appreciable muscle weakness exists when the horse tries to use the affected limb or limbs.

A myopathy is characterised by muscles that are swollen, firm to the touch, painful on palpation and non-functional. Muscle fasciculations are sometimes visible and the horse often shows distress due to pain. Myocyte rupture results in elevated blood creatine kinase, which can be variable depending on the extent of myocyte damage.
Muscle swelling can cause secondary nerve compression, resulting in a combination of myopathy and neuropathy to exist. Radial or femoral nerve paralysis can occur due to focal areas of pressure or inappropriate traction on the affected limb.
Facial nerve paralysis can result after focal or uneven pressure is exerted over the masseter muscles. Most commonly, this occurs in horses positioned in lateral recumbency with inadequate or uneven padding, or nerve injury caused by a head collar buckle.
Head collars should be removed before the horse is positioned in lateral recumbency. Alternatively, custom-made head collars are available that are free of protruding buckles. If placement of a head collar is unavoidable, the buckles or any feature that exerts uneven pressure should be well padded.
The treatment of myopathy/neuropathy is supportive and includes IV fluids, correction of electrolyte imbalances, administration of acepromazine to reduce anxiety and promote peripheral tissue blood flow via peripheral vasodilation, analgesics and anti-inflammatories.
It may not always be possible to move the horse from the recovery box, and supportive treatment should be initiated in the recovery box until the horse is able to safely walk to the stable. In some cases, the onset of clinical signs of myopathy is delayed, and the horse may recover and walk back to the stable with little clinical suspicion of myopathy until clinical signs develop several hours later. In these cases, tissue ischaemia and subsequent reperfusion injury may be involved.
In many cases of myopathy/neuropathy, a clinical improvement occurs within 12 to 24 hours after initiation of supportive treatment. However, in very severe cases, or when multiple muscle groups are affected, the horse may remain recumbent for several days and require intensive nursing care.
Spinal cord malacia, spinal cord degeneration and post-anaesthetic myelopathy are terms used to describe clinical signs of loss of voluntary function in the hindlimbs that become apparent during the recovery phase from general anaesthesia.
Ragle et al (2011) reported a case series of 30 horses with post-anaesthetic myelopathy, 26 of which had been positioned in dorsal recumbency, 24 were male and the median age was 14 months. Affected horses were mentally alert during the recovery phase, but showed flaccid paralysis, areflexia and analgesia of the hindlimbs, loss of anal and tail tone, and lack of panniculus response (Ragle et al, 2011).
Affected horses tend to “dog-sit” and become increasingly anxious. Spinal cord degeneration and necrosis resulting in poliomyelomalacia is seen histopathologically – and while these changes may be most consistent with ischaemic injury, the aetiology remains unknown and is likely multifactorial.
In the case series reported by Ragle et al (2011), despite supportive therapy, no improvement in neurological function occurred and all individuals died or were subjected to euthanasia from a few hours to eight days postoperatively.
If controlled mechanical ventilation has been employed during general anaesthesia, a period of apnoea may occur in the early recovery phase before the horse resumes spontaneous breathing.
Earlier return to spontaneous breathing may be encouraged by slightly reducing the respiratory rate delivered via mechanical ventilation in the final few minutes of general anaesthesia to ensure that end‑tidal carbon dioxide tension is not too far below the threshold at which respiratory drive is stimulated.
Continued intermittent positive pressure ventilation can be employed using a demand valve and as soon as the horse resumes spontaneous breathing, the endotracheal tube cuff should be deflated to avoid increased inspiratory and expiratory resistance developing.
Nasal insufflation of oxygen can also be performed after the endotracheal tube has been removed. Hypoxaemia in the recovery period caused by ventilation and perfusion mismatching usually resolves when the horse attains the sternal position, and furthermore when the horse returns to standing.
Pulmonary oedema is the abnormal accumulation of liquid and solute in the extravascular tissues and spaces of the lung (Senior, 2005). Lung volume and elasticity is reduced, and alveolar surfactant function becomes compromised, which reduces lung compliance further.
Gross disruption of the gas exchange interface promotes hypoxaemia and cellular hypoxia, which can be severe enough to progress to cerebral and myocardial hypoxia, resulting in death.
Negative pressure pulmonary oedema can result from the generation of excessive negative intrathoracic pressures caused by inspiratory efforts against a closed glottis or obstructed upper airway. Bilateral laryngeal paralysis or complete nasal occlusion can result in negative pressure pulmonary oedema.
Horses experiencing any degree of airway obstruction or difficulty are likely to become very anxious and agitated, and elevated circulating catecholamines may have a role in altering pulmonary capillary integrity and pulmonary vascular resistance. In the recovery period, increased respiratory stertor or stridor may be an indication of respiratory obstruction and intervention may be warranted.
Nasotracheal tubes can be routinely placed in horses recovering from general anaesthesia to reduce any airway resistance caused by nasal oedema. If the tube is not secured in place, the horse usually dislodges the tube once sternal recumbency is attained, by which time nasal oedema may be improved.
The nasotracheal tube can be secured in place and removed once the horse is standing or further intervention such as a tracheostomy has been carried out to alleviate upper respiratory obstruction that is not transient in nature.
Regardless of the exact cause, pulmonary oedema can develop rapidly and have fatal consequences in a short space of time.
The emergency nature of the situation can be exacerbated by the appearance of the clinical signs of pulmonary oedema at the time when the horse is in the early stages of regaining consciousness from anaesthesia and limb movement has already started to occur, together with attempts to try to rise. The timing of these events certainly poses a hazard to anyone attempting to intervene at this stage.
In the event of airway obstruction, the primary aim is to restore airway patency, either using an endotracheal tube, nasotracheal tube or tracheostomy tube.
Oxygen supplementation and anxiolysis is also useful once a patent airway is achieved.
Acepromazine administration may be useful as a tranquilliser and acts to reduce pulmonary vascular resistance. Other treatments include furosemide, NSAIDs, glucocorticoids and bronchodilators (Senior, 2005).
Fractures have been described as responsible for 26% to 64% of all anaesthesia-related fatalities, and this increased to 71% when dislocations were included (Dugdale and Taylor, 2016). Assisting a horse safely to the standing position after general anaesthesia is difficult to consistently achieve and while a range of assisted recovery methods exist, no overwhelming evidence exists to indicate that any one method is superior.
A horse’s temperament and the nature of the surgical procedure may affect the recovery quality and advancing age (older than 14 years), the presence of comorbidities, the potential presence of osteoporosis in broodmares, and systemic and muscular fatigue in older horses recovering after undergoing colic surgery may be factors that influence the occurrence of fractures or joint luxation in recovery (Dugdale et al, 2016).
Long bone fractures or limb dislocations can occur even when the nature of the recovery is calm. An unevenly weighted step or stumble can result in a catastrophic injury. Keeping the recovery environment noise to a minimum, avoiding slippery flooring by drying any sweat, and catheterising and emptying the urinary bladder prior to recovery can aid in improving recovery safety, and prevent premature attempts to stand.
The incidence of post-anaesthetic colic in elective cases varies from 2.8% (Senior et al, 2006) to 10.5% (Jago et al, 2015). Implicating factors may include altered management of the horse associated with hospitalisation, such as transport, dietary and environmental change.
Nelson et al (2013) identified some specific risk factors for the development of postoperative colic, including the Arabian horse breed, elevated intraoperative peripheral lactate, right lateral recumbency during general anaesthesia, decreased post‑anaesthetic rectal temperature and delayed passage of faeces.
Close observation of clinical parameters and documented passage of faeces (volume and consistency) in the postoperative period is important in monitoring gastrointestinal function. Further investigation of colic signs should be carried out promptly if any suspicion of gastrointestinal dysfunction exists. Most cases of postoperative colic are responsive to medical management.