12 Jan 2021
Hayley Chidlow provides clear advice for fellow vets, with particular focus on vaccination protocols.

Equine influenza (EI) is a major cause of equine respiratory disease worldwide, and is highly contagious.
The virus is endemic in the UK, as well as the rest of Europe and North America, and normally circulates at a low level. Prior to 2019, the annual number of EI outbreaks in the UK had been trending downwards, with only two outbreaks in 2018. However, a dramatic increase in EI outbreaks was observed in 2019, with 228 laboratory confirmed outbreaks1.
The increased prevalence of EI in 2019 was attributed to a number of factors, including low vaccination rates of the general equine population in the UK, antigenic drift of the virus resulting in a lack of homology between the field virus and vaccine strain, and increased virulence of the circulating EI strain resulting in increased clinical disease.
Influenza viruses are divided into subtypes according to their surface glycoproteins – haemagglutinin (HA) and neuraminidase (NA) – which play a vital role in pathogenesis. HA is responsible for binding of the virus to the cell surface, and subsequent fusion between virus and cell membrane. Following virus replication, neuraminidase then cleaves the newly made viruses from the infected cell surfaces, allowing these progeny viruses to disperse and infect more cells.
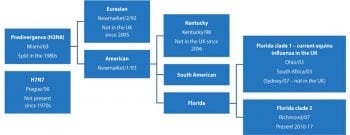
EI is caused by two subtypes of influenza A viruses – H7N7 and H3N8. Currently circulating EI viruses belong to the H3N8 subtype; the H7N7 subtype has not been isolated from cases of EI in more than 30 years. As with human influenza viruses, EI surface glycoproteins accumulate point mutations that result in antigenic drift. This has resulted in divergence of the EI H3N8 viruses (Figure 1)2.
In the late 1980s, continual antigenic drift resulted in formation of two separate H3N8 lineages, European and American3. The European strain has not been isolated in the UK since 2005.
The American lineage has subsequently split into distinct sublineages, Kentucky and Florida. The H3N8 Florida sublineage has been responsible for all EI outbreaks in the UK since 2006, and is now classified as either clade 1 or clade 2.
Both clade 1 and clade 2 viruses currently circulate in the UK and Europe, and are an ongoing threat. Clade 2 strains were isolated in EI outbreaks in the UK from 2010-17, while clade 1 strains were responsible for outbreaks in the UK in 2018 and 20194.
In unvaccinated horses, clinical signs of EI include a dry cough, pyrexia and serous or mucoid nasal discharge. Affected horses are often lethargic, with a decreased appetite. Coughing, in particular, is a prominent feature of EI compared to other infectious respiratory diseases, and results in substantial virus shedding into the environment.
Aerosolised influenza virus particles can spread up to 2km. In combination with a short incubation period, this means that in a naive population EI will spread rapidly and infection rate may approach 100%. Vaccinated horses can be asymptomatic or show only mild signs of respiratory disease, and even those horses with partial protection due to irregular vaccination will generally show milder clinical signs.
Timely and accurate diagnosis of infected horses is a vital part of controlling EI outbreaks. Rapid diagnosis can be achieved by collecting nasopharyngeal swabs and submitting for real-time PCR testing. Turnaround time using this diagnostic method can be less than 24 hours from submission.
Paired serum samples, collected not less than 14 days apart, should also be submitted. In an outbreak scenario, unvaccinated horses with compatible clinical signs should be tested. Vaccinated horses with non-specific respiratory signs should also be tested. In small groups, testing of all unvaccinated in-contacts may be feasible, but large-scale testing of asymptomatic horses on large yards is not recommended5.
The World Organisation for Animal Health (OIE) is responsible for disease surveillance, with several reference laboratories around the world, which included the AHT in the UK. The Horserace Betting Levy Board (HBLB) has a surveillance scheme that allows free testing of suspected EI cases. Information on UK outbreaks is then distributed by several sources, including Equiflunet (www.equiflunet.org.uk) and Tell-Tail (sponsored by Boehringer Ingelheim; www.telltail.co.uk).
The ability of a vaccine to reduce viral transmission depends on a number of factors, including the time since last vaccination, antigenic similarity between the infecting virus and the vaccine strain, the immune response to vaccination and the number of in-contacts6. As a result of antigenic drift, vaccine strains must be updated regularly to provide adequate protection.
OIE reference laboratories are responsible for tracking data on EI cases and vaccine breakdown, and characterising the viruses that are isolated. This includes analysing genetic data to assess whether current EI isolates differ antigenically from vaccine strains. The OIE Expert Surveillance Panel (ESP) meets annually to make recommendations regarding EI vaccine composition. The most recent change to vaccine recommendations occurred in 2010, when the ESP recommended that vaccine products included representative strains from both Florida clade 1 and clade 23.
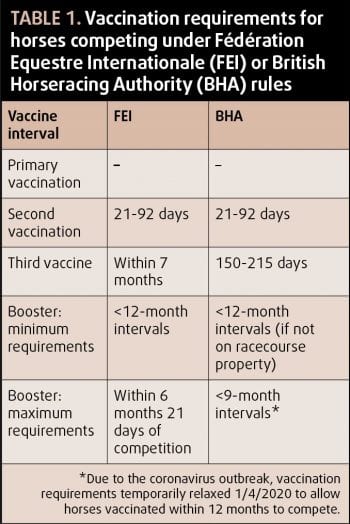
Of the three UK-licensed vaccine brands, ProteqFlu (Boehringer Ingelheim) is compliant with the current OIE ESP recommendations (Table 1). Equilis Prequenza (MSD Animal Health) contains Florida clade 1 but not clade 2, thereby complying with OIE ESP recommendations from 2004. The remaining vaccine brand (Equip F, Zoetis) contains neither of the Florida sublineage strains, and is therefore only compliant with the 1995 OIE ESP recommendations2.
The vaccination protocols recommended by the major regulatory bodies in the UK are outlined in Table 2. Some discrepancy exists between recommended intervals of the second vaccination and first booster between manufacturers and regulatory bodies. Most vaccine manufacturers recommend a four-week to six-week interval between first and second vaccination, and five to six-month interval between second vaccination and first booster.
While it is important for competing horses to adhere to regulatory body recommendations, it is also important to follow data sheet recommendations to ensure maximal efficacy of the vaccine and prevent vaccine breakdown. If the maximal interval between vaccines, as allowed by regulatory bodies, is followed, then gaps in immunity may occur due to low antibody levels7,8.
Unvaccinated horses shed large viral loads, resulting in a short incubation period and rapid transmission through the herd (Figure 2). Vaccinated horses may still become infected; however, they have reduced viral shedding and a decreased length of infection compared to unvaccinated horses and will often be asymptomatic or have only mild clinical signs9,10.
Owners often mistakenly believe that as long as their own horse is vaccinated, the vaccination status of the rest of the herd does not matter. Prior to the 2019 outbreaks, it was estimated only 30% to 40% of the UK horse population was vaccinated – well below the 70% to 80% required to achieve herd immunity. A common scenario in EI outbreaks is the introduction of the virus to a yard by a vaccinated, asymptomatically shedding competition horse.
A review by the OIE found that 88% of EI outbreaks occurred following movement of subclinically affected horses to/from regulated events11. If the virus is introduced into a fully vaccinated population, viral shedding will remain low; therefore, transmission of the virus will be minimised. In comparison, if the virus is transmitted to an unvaccinated horse, it will be amplified resulting in substantial virus shedding and rapid spread of the infection through the population.
If viral loads are sufficient, other vaccinated horses on the yard can become infected – especially if they are in close proximity to unvaccinated cases12.
A major deciding factor in whether vaccination can be employed as part of an outbreak control strategy is the timing of detection of the outbreak. Vaccination is not recommended in horses that have already developed clinical signs; therefore, early detection is critical.
In a small herd with close contact between horses, detection of an outbreak early enough for vaccination of in-contact animals to be effective may be difficult, and as long as the population is otherwise healthy, it may be simpler to allow the infection to move through the group.
In large groups of horses, the amplification effect of unvaccinated individuals in the group will result in more rapid spread and increased morbidity; therefore, vaccination of in-contacts is more beneficial from both an animal welfare and economic perspective. In populations containing very young or elderly equids, or those with other health problems, an increased risk of severe or even fatal clinical disease exists. Vaccination of in-contacts is particularly important in these scenarios to reduce circulating viral loads.
As well as vaccination of all asymptomatic, unvaccinated individuals, vaccination of previously vaccinated horses should be considered. Some horse owners may query why their horse requires a booster when it is fully vaccinated. This brings us back to the factors that affect a vaccine’s ability to reduce viral transmission: time since last vaccination, antigenic similarity between the infecting virus and the vaccine strain, the immune response to vaccination and the number of in-contacts.
In a study investigating EI outbreaks in Ireland, 34% (27 of 118) of vaccinated horses developed clinical signs6. Of these horses, 67% had not had a booster vaccination within the prior six months, and 37% were due their annual booster vaccination.

Therefore, in an outbreak situation, vaccinating in-contacts that have not received a booster vaccine within the six months prior to the outbreak is recommended to reduce vaccine breakdown. Likewise, if the horse was previously vaccinated with a product containing outdated vaccine strains then booster vaccination with a product that meets OIE ESP recommendations is recommended.
Experimental studies have shown the recombinant canary pox vaccine (ProteqFlu) reduces viral shedding and clinical signs after one dose13; therefore, even a single vaccination in the face of an outbreak can reduce both individual morbidity and help mitigate spread of the virus within the herd.
ProteqFlu meets current OIE ESP recommendations, and contains both Florida clade 1 and clade 2 strains. Given all outbreaks within the past 5 years have been caused by clade 1 and clade 2 viruses, this vaccine is likely to provide the best protection in the event of an outbreak on both a herd and individual level.
However, if this vaccine is unavailable during an outbreak situation then some protection is still provided by either of the other UK-licensed products.
As well as addressing vaccination on the affected yard, consideration must be given to instituting vaccination policies on properties surrounding an infected case – particularly in areas heavily populated with equestrian properties.
Following the 2007 EI outbreak in Australia, simulation studies demonstrated a significant reduction in the size of the outbreak when an early vaccination protocol was used. A 1km ring of vaccination around a property was found to be most effective when vaccine availability was limited, while a 3km ring was more effective when vaccine capacity was increased.
In a UK outbreak situation, serious consideration should be given to instituting a suppressive ring vaccination policy to reduce the number of new infections and the size of the infected area9,14.
Vaccination can play an important role in control of EI outbreaks, and should be considered for reduction of morbidity in individual animals and mitigation of spread between animals on the infected premises and to other premises.
When deciding whether to institute a vaccination policy in an outbreak, factors include early detection to allow vaccination to be commenced in asymptomatic in-contacts. Populations of unvaccinated horses or yards with mixed vaccination status are most at risk of rapidly spreading outbreaks. It is important to remember vaccination alone will not control an outbreak, and it should be used in combination with biosecurity, including isolation of affected horses and movement controls on and off the premises.