7 May 2018
Andy Durham discusses surveillance schemes and clinical choices for this condition, and lists some of the more recent outbreaks.

Would you habitually take swabs for influenza from horses with laboured breathing or snotty noses?
Through the summer and autumn of 2017 several equine influenza outbreaks were diagnosed in Lancashire, Yorkshire, Northumberland and Essex.
It is important to remember these diagnoses may well represent the tip of the iceberg, due to undertesting of possible influenza cases. Not all of us are inclined to submit diagnostic samples for testing for influenza very frequently despite seeing coughing or pyrexic horses on a regular basis.
We should also be mindful many horses are still vaccinated with products containing outdated strains of influenza virus or may be irregularly vaccinated, leading to milder, non-specific respiratory signs that may not always trigger thoughts of influenza.
A free equine influenza surveillance scheme is run by the AHT and funded by the Horserace Betting Levy Board (HBLB). A very simple registration form for the scheme can be completed online1, whereupon sampling packs will be sent out.
Practices or individual practitioners are encouraged to register then submit samples from suspect cases that they might see comprising horses from the following categories:

Additionally, immediate notification about outbreaks of influenza (and equine herpesvirus abortion/neurological disease) can be obtained by the free Tell-Tail service developed by Merial Animal Health (part of Boehringer Ingelheim Animal Health). When outbreaks have been diagnosed in the UK, an SMS text message is sent to all registered participants2 informing them of the disease diagnosed and the geographical location.
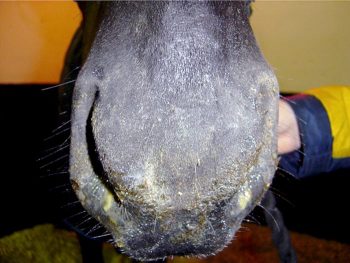
Given the reasonably high prevalence of vaccination among horses where veterinary attention will be sought following respiratory signs, it is important to bear in mind it is likely to be horses showing non-specific respiratory signs that may prove to be the most common target for this scheme. Thus, horses showing nothing more than mild serous nasal discharge, pyrexia of undetermined origin, laboured breathing patterns, the occasional cough or submandibular lymph node reaction should all be considered as candidates for the testing scheme (Figure 1).
As with most infectious diseases, diagnosis can be made in two ways: by identifying the presence of the infecting microorganism itself and/or by identifying a serologic response to it. Ideally, the former means is preferable as this provides both irrefutable evidence of infection, and gives opportunity to isolate and characterise the specific virus strain involved.
Nasopharyngeal swabs (Figure 2) should be collected as soon as possible after onset of signs, as influenza virus shedding tends to be fairly short-lived and no longer than a few days. When signs have already been present for more than four or five days, some usefulness might still be gained from swabbing in-contacts that might be at an earlier stage of infection.
Serologic response is also useful to indicate viral exposure where virus isolation or identification has not been possible. Given the strong possibility of previously circulating influenza antibodies in many horses, demonstration of seroconversion is required and necessitates two plain serum samples to be collected – one as early as possible in the disease course then the second sample no less than two weeks later.
In addition to informing clinical diagnosis, the information collected from swabs submitted by practitioners is also crucial in following the evolution of circulating virus strains in the UK and abroad, and also comparing the circulating strains with those used in commercial vaccines. This data is used to determine whether strain recommendations should be updated or not. It is concerning several outbreaks are reported in the UK, Ireland and further afield in horses fully up to date with their influenza vaccine courses, highlighting the inadequacies of the protection offered by several of our choice of vaccines.
Equine practice is full of clinical choices we make based on experience, published evidence or recommendation. Many pharmaceutical brand choices are arguably closely similar, and certainly many share almost identical key ingredients, making the choice less impactful. However, when it comes to vaccine choice indisputable major differences occur between vaccine brands, and in my experience, all are accompanied by manufacturer representatives offering a convincing story why their one is best.
However, equine influenza vaccine choice is probably unique as an annually updated and concise document – put together by a panel of independent, internationally renowned experts who review available evidence and offer recommendations – is free and readily available to access.
This document published by the World Organisation for Animal Health (OIE) Expert Surveillance Panel (ESP) on Equine Influenza Vaccine Composition, is available from the OIE website in spring every year.
The most recent issue was in March 2017 and can be found online3. Practising vets willing to sample suspect cases in the UK via the HBLB scheme (and practitioners collecting samples in other countries) play a crucial role in informing the panel, with submission of clinical samples to reference laboratories being an essential part of monitoring antigenic and genetic drift within equine influenza viruses. Vets in practice are also key for surveillance and investigation of vaccination breakdown.
Nevertheless, the circulating viruses all remain antigenically closely related to the recommended vaccine strains for Florida clade one (Ohio, 2003, South Africa, 2003) and Florida clade two (Richmond, 2007) viruses.
Pertaining to the UK, the ESP comment that although a single UK vaccine has been updated to include a virus from Florida clade two, in accordance with the recommendations of 2010 to 2016, many vaccines still contain outdated strains and “the updating of vaccines with epidemiologically relevant viruses is necessary for optimum protection”. The unedited recommendations of the ESP are detailed in Panel 1.
For several years, the greatest equine influenza threat to UK horses has consistently come from Florida clade two viruses. Inclusion of a representative strain (Richmond, 2007-like viruses) in vaccines for the UK market was first recommended by the ESP in 2010 and this recommendation has been repeated every year since. Disappointingly, however, only a single vaccine brand has complied with this expert recommendation.
Florida clade two viruses are the clear major threat to UK horses and yet only some of us choose a vaccine containing this strain.
The ongoing use of vaccine brands electing to ignore the unequivocal advice of the ESP is, in my opinion, not an ideal situation, howsoever brand marketing might try to convince otherwise.