27 Mar 2018
Nicola Menzies-Gow discusses a slowly progressive neurodegenerative disease with loss of dopaminergic (inhibitory) input to the melanotropes of the pituitary pars intermedia.
Equine pituitary pars intermedia dysfunction (PPID) is a slowly progressive neurodegenerative disease with loss of dopaminergic (inhibitory) input to the melanotropes of the pituitary pars intermedia, which appears to be associated with localised oxidative stress and abnormal protein (α−synuclein) accumulation. However, the exact cause remains unknown.
The consequent dysfunction of this region results in hyperplasia of this area of the gland, and overproduction of pars intermedia-derived hormones. Eventually, the area undergoes adenomatous change.
The condition is seen in older animals; the average age in retrospective case series ranges from 18 to 23 years1-4. It has no breed or sex predilection, but ponies are more frequently affected than horses in some studies2-4. In one survey, 21% of horses aged 15 years or older had endocrine changes consistent with PPID5 and, in a second, clinical signs consistent with PPID were documented in nearly 40% of horses aged above 306.
However, it should be noted, the disease is recognised in animals younger than this, with the youngest reported case being in a seven-year-old animal7.
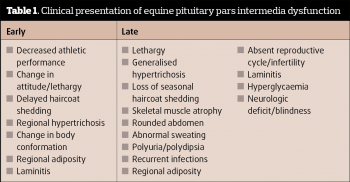
Clinical signs associated with PPID can be roughly divided into those seen early in the disease and those associated with advanced disease (Table 1). PPID diagnosis is based on signalment, clinical signs and further diagnostic test results. Discussion of these further diagnostic test results will form the focus of the remainder of this article.
No ideal further diagnostic test for equine PPID is available. However, plasma basal adrenocorticotropic hormone (ACTH) concentrations and the ACTH response to thyrotropin-releasing hormone (TRH) are thought to be the most appropriate tests available for equine PPID (Figure 1).
Each of these tests will be assessed in turn in relation to their ability to provide accurate diagnostic information to the equine practitioner.
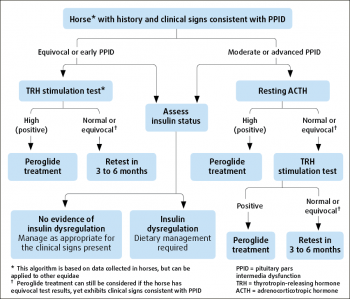
Two areas relating to plasma ACTH concentrations potentially create difficulties when this test is used to make a diagnosis of equine PPID, namely the cut-off value and the assay used to measure ACTH concentration.
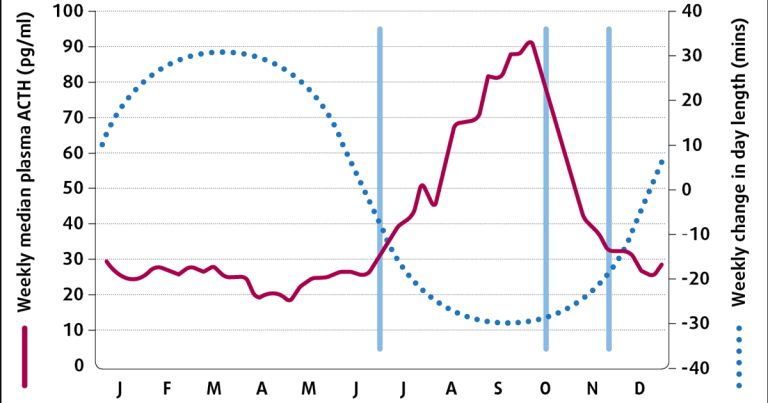
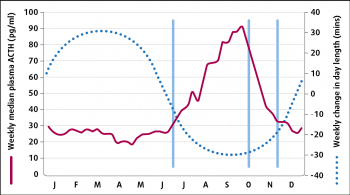
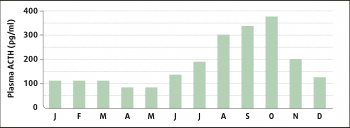
Plasma ACTH concentrations vary with the season, with a rise spanning July to November in the UK – corresponding to the change in day length (Figure 3)10.
Throughout the year, plasma ACTH concentrations are greater in animals with PPID compared to normal animals and this autumnal rise is greater in animals with PPID compared to normal horses11. Therefore, it is essential a seasonally adjusted reference range is used when interpreting ACTH concentrations. However, the cut-off value recommended consistent with a diagnosis of PPID varies between publications (Table 2).
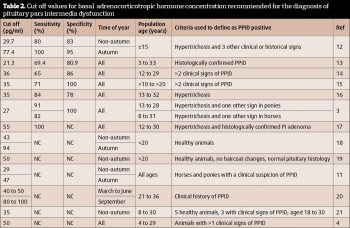
Suggestions for the non-autumn period range between 27pg/ml and 50pg/ml, while suggestions for the autumn period range between 47pg/ml and 100pg/ml.
The sensitivity (correctly detects a positive result) and specificity (correctly detects a negative result) of these cut-off values is only reported in a subset of these publications. In those publications where season is not adjusted for, the sensitivity and specificity values range from 65% to 100% and 78% to 100%, respectively.
The lowest values in these ranges equate to an incorrect test result in either the positive or negative direction occurring in approximately 3 out of every 10 tests performed. False positives occur due to physiological increases in ACTH concentration, such as severe pain, strenuous exercise and moderate to severe illness22. False negatives are thought to occur due to the poor ability of the test to detect early disease.
The sensitivity and specificity of the test do not appear to be improved by measuring ACTH concentrations in paired samples13. However, measuring ACTH concentrations in the autumn using a seasonally adjusted cut-off value does appear to result in a significant improvement in these values. In the publication that determined the sensitivity and specificity of autumn (77.4pg/ml) and non-autumn (29.7pg/ml) cut offs, sensitivity and specificity values of 80% and 83%, and 100% and 95%, respectively, were reported.
It should be noted the studies used to determine these values vary in criteria used to define an animal as PPID positive, and also that the majority of studies required the presence of hypertrichosis to classify an animal as positive for PPID. Therefore, although it would appear it is best to measure plasma ACTH concentrations in the autumn, the sensitivity and specificity of plasma ACTH concentration for the diagnosis of PPID animals without hypertrichosis, regardless of the time of year, is less certain.
It has been suggested by some authors that, rather than a specific cut off, instead a grey zone of ACTH concentrations should be between 19pg/ml to 40pg/ml23 or 30pg/ml to 50pg/ml (Equine Endocrinology Group) in the non-autumn months, and 50pg/ml to 100pg/ml (Equine Endocrinology Group) in the autumn.
Results falling within these zones should be considered as equivocal, requiring testing to be repeated or a TRH stimulation test to be performed. However, the sensitivity and specificity of these grey zones has yet to be determined.
As well as the autumn rise, it would appear a nadir occurs in plasma ACTH concentration in April24. Therefore, an alternative diagnostic cut off may also be required for April. Finally, the size of the autumn rise in plasma ACTH concentration appears to vary, with horse-related factors including breed, sex and coat colour25. As an example, greater rises occurred in Shetlands, mares and grey-coloured animals. Consequently, the cut-off values recommended for a diagnosis of PPID may additionally need to vary according to horse-related factors. All of these variables require further investigation.
More than one method is validated for the measurement of ACTH concentration in equine plasma samples. The method used by the majority of the UK’s commercial laboratories is the chemiluminescence (CL) method. Other available validated methods include an immunofluorescence (IF) method.
It has been reported the results obtained from the CL and IF methods do not agree, with results using the IF methods being lower than those obtained using the CL method (median difference 29.9pg/ml)26. Cut-off values recommended for the diagnosis of PPID should, therefore, be specific to the assay.
In addition, it would appear ACTH degradation products, such as corticotropin-like intermediate peptide, cross-react with the CL assay or interfere with the IF assay26. Thus, the CL method may be measuring other pars intermedia-derived pro-opiomelanocortin (POMC) products, as well as ACTH in equine plasma. Whether this cross-reaction is useful when making a diagnosis of equine PPID has yet to be determined.
TRH is a physiologic release factor for the equine pituitary, so its administration stimulates release of the normal hormone products from the pars intermedia. The ACTH response to TRH is measured. A dose of 0.5mg, 1mg or 2mg TRH produces ACTH responses that are not significantly different27,28.
The sensitivity and specificity of the test in animals with two or more clinical signs consistent with PPID, using a cut-off value of 36pg/ml, after 30 minutes in three studies were 94% and 78%, respectively14, 88% and 71%, respectively29, and 77% and 82%, respectively15. Therefore, if using the lowest of these values, the test offers an incorrect result in either the positive or negative direction in approximately 2 out of every 10 tests performed.
It should be noted these sensitivity and specificity values were derived for ACTH concentrations measured 30 minutes after the administration of TRH. It is more common to measure ACTH in samples obtained 10 minutes after TRH administration and similar values have not been published for this time point.
The test is affected by feeding, so animals should be tested under short-term fasted conditions or not receive supplementary grain feed prior to testing30, nor should the test be performed immediately after an oral sugar test (OST)31. In addition, the test is affected by season24,32-34 (Figure 4), and seasonally adjusted cut-off values should be used for the interpretation of the result; however, the sensitivity and specificity for these have yet to be published. As a result, it is preferable to avoid performing the test during the autumn rise in pituitary activity, if at all possible.
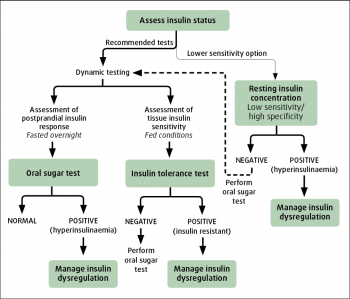
ID in equines encompasses hyperinsulinaemia, an excessive insulin response to oral carbohydrate and tissue insulin resistance. Tests should be employed to determine the presence or absence of each to aid a decision on whether an animal with PPID has ID.
It was previously suggested basal insulin concentrations should be measured in fasting animals. However, it is recommended concentrations are measured in animals that have not been fed grain within four hours to detect hyperinsulinaemia.
Previous recommendations of serum insulin concentrations lower than 20μIU/ml being consistent with ID were based on the animal being fasted35. It is suggested a serum insulin concentration lower than 20μIU/ml is not diagnostic, and a dynamic test should be performed; that a value between 20μIU/ml and 50μIU/ml is suspicious of ID, but a dynamic test should be performed; and that a value greater than 50μIU/ml is consistent with ID (Equine Endocrinology Group). However, it should be noted numerous factors influence serum insulin concentrations, and the sensitivity and specificity of these cut-off values have not been determined.
In addition, many validated assays are available for the measurement of equine serum insulin concentrations. While the chemiluminescence (CL) assay is commonly used by commercial laboratories in the UK, radioimmunoassays and ELISA are additionally used in research studies.
The values obtained by the various assays are not identical. For example, values obtained using the CL assay are consistently lower than those obtained using radioimmunoassays36,37. Cut-off values should be specific to the assay used to generate that value, and are, thus, not universally applicable.
An OST using Karo Light corn syrup (0.15ml/kg to 0.45ml/kg) or an OGT using glucose powder (0.5g/kg to 1g/kg) can be performed to detect an excessive insulin response to oral carbohydrate.
These can be performed either after a three-hour to eight-hour fast or while the animal remains at pasture38. Serum insulin concentrations are measured after 60 to 90 minutes (OST) or 180 minutes (OGT). An insulin concentration greater than 60μIU/ml (0.15ml/kg OST), greater than 110μIU/ml at 60 minutes (0.45ml/kg OST) or 85μIU/ml at 180 minutes (1g/kg OGT) is considered consistent with ID.
In one study, the OST and OGT agreed, with respect to binary classification of animals as ID or insulin sensitive, in 85% of animals39. However, in another study, the repeatability of the OST was poor, with the within-subject coefficient of variation (cv) of the OST at any single time point being 32% when animals remained at pasture or 40% when animals were starved38.
The repeatability of the OGT was fair, with the median cv of the insulin concentration at 120 minutes being 19%40. In addition, the OST and OGT are affected by season, with greater OST insulin responses occurring in summer compared to winter and greater OGT insulin responses occurring in autumn and winter compared to spring and summer41. Finally, it should be remembered the sensitivity and specificity of these cut-off values have not been reported, and these cut-off values are specific to the assays used in the studies to measure serum insulin concentrations.
The euglycaemic hyperinsulinaemic clamp (EHC) method is the gold standard technique to determine tissue insulin resistance. However, it is not practical to perform in the clinical setting. Instead, an insulin response test (IRT) is recommended for the detection of tissue insulin resistance.
A dose of 0.1 IU/kg regular (soluble) insulin is administered IV and the blood glucose concentration measured after 30 minutes. In the single study reporting its use, blood glucose concentrations decreased by more than 50% from baseline in all six normal horses, while the decrease was under 50% in all six horses with tissue insulin resistance42.
Horses should not be fasted when performing an IRT, as fasting decreased the response to insulin43. It should be acknowledged the IRT has not been compared to the EHC, thus its sensitivity and specificity is unknown, and its repeatability has not been reported.
As well as the now well-documented effect of season on the output of hormones from the equine pituitary pars intermedia, several other possible reasons account for the less than ideal sensitivity and specificity of the tests available.
Firstly, a lack of histological diagnosis consensus exists for PPID44 – thus, no gold standard against which antemortem tests can be validated. Secondly, PPID is a progressive disease and diagnostic tests appear to have greater accuracy when applied to cases with more advanced clinical signs. Their sensitivity and specificity in early cases with clinical signs that do not include hypertrichosis have yet to be adequately determined.
Finally, ACTH is not one of main products of the equine pituitary pars intermedia. Thus, greater diagnostic accuracy may be achieved by measuring alternative, as yet undetermined, hormones or even the plasma POMC peptide signature.