24 Oct 2016
Andy Durham discusses forms of protection against illnesses in horses, such as tetanus and equine arteritis virus.

 Vaccination is commonly employed in the UK equine industry as part of the approach to limiting equine influenza and tetanus. Additionally, some horses receive vaccines designed to offer protection against infection, with equine arteritis virus, equine herpesviruses (EHV) 1 and 4, rotavirus and, rarely, West Nile virus (WNV).
Vaccination is commonly employed in the UK equine industry as part of the approach to limiting equine influenza and tetanus. Additionally, some horses receive vaccines designed to offer protection against infection, with equine arteritis virus, equine herpesviruses (EHV) 1 and 4, rotavirus and, rarely, West Nile virus (WNV).
However, when did you last encounter a horse affected by one of these diseases? Most of us in equine practice see affected horses infrequently or not at all, which tends to breed complacency and, perhaps, creates a perception that devalues the significance of our efforts to maintain a good and effective vaccination policy.
Vaccination should never be a substitute for other good biosecurity measures (both national and regional), but at least it adds another level of security for relatively little effort and cost.
Ever-evolving strains of equine influenza are frequently isolated from UK horses and represent an ever-present danger.
However, even equine influenza vaccination, designed to protect against the most relevant and significant threat among the diseases listed previously, is administered to fewer than half of the horses in the UK, despite this being mandatory in many equestrian activities. This is far lower than the estimated minimum population coverage required to control spread should an epidemic threaten to arise.
The possible welfare and economic consequences of low vaccination coverage were clearly illustrated by the South African and Australian equine influenza epidemics in 2003 and 2007, whereby devastating damage occurred in a partially or entirely unprotected equine population. Similarly, the unexpected – and, largely, unexplained – arrival of WNV in New York in 1999 wreaked havoc in a national US herd of immunologically naïve horses.
Although, thus far, WNV has not been officially recognised any closer to us than southern France and northern Italy, no logical reason exists as to why it might not arrive here and, indeed, there is probably greater reason to expect it to arrive in the UK than our US colleagues would have predicted in advance of its arrival on US shores.
Four equine-licensed influenza vaccines are available in the UK (Duvaxyn, Elanco; Equip F, Zoetis; ProteqFlu, Merial; Equilis Prequenza, MSD). Each is significantly different from the others, meaning practitioners have an important choice to make when selecting a product on behalf of their clients.
Ideally, one would hope this choice is made on a well-considered scientific basis rather than commercial decision, but, unfortunately, given the reality of busy equine practice, this might not always be the case, especially given careful and selective marketing material presented by vaccine manufacturers.
The most fundamentally important element of vaccine products is the antigenic material in them. Only one product is available in the UK containing both recommended circulating influenza strains relevant to UK horses (ProteqFlu) and, in the author’s view, other manufacturers have shown little enthusiasm for investing in updating strains to deal with the modern threat from Florida clade 2 strains, especially while they maintain significant sales of outdated vaccine through UK vets.
It appears commercial pressure from our own profession as the direct consumers of vaccine products is likely to have more impact than the recommendations from the independent World Organisation for Animal Health (OIE) expert surveillance panel (OIE, 2016).
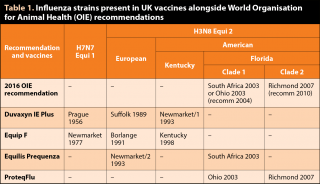
ProteqFlu fully complies with the latest (March 2016) recommendations and Equilis Prequenza complies with the OIE recommendation made in 2004, while Duvaxyn IE and Equip F only comply with the 1995 recommendations (Table 1).
Concerns are often raised regarding the fact that despite significant antigenic differences between vaccine products, it is very common individual horses are vaccinated with different vaccine brands, which would appear intuitively suboptimal from an immunologic perspective.
A study of 102 Thoroughbreds in training in Ireland confirmed 95% of them were vaccinated with more than one vaccine brand (Ryan et al, 2015). It is recommended by manufacturers that Duvaxyn IE or Equip F are not used interchangeably with other vaccine brands and a new primary course is started should brand use change.
Interestingly, in this study, horses vaccinated with different vaccine products demonstrated significantly higher antibody responses than those vaccinated with a single product, perhaps contrary to expectations. However, this may have been a consequence of the particular vaccine brands being compared.
Clearly, it would be expected a simple vaccine containing inactivated whole virus combined with a double adjuvant of aluminium hydroxide and Carbopol is likely to result in higher circulating antibody levels than other vaccine products engineered to generate cell-mediated and mucosal antibody responses.
Furthermore, it should be remembered circulating antibody levels are an unreliable predictor of protection against influenza, which is generally a non-viraemic disease.
Tetanus toxoid, either alone or in combination with influenza, is an effective means of preventing tetanus in horses.
Duration of immunity remains unknown in horses, although booster vaccinations every two to three years are generally recommended, with further boosters when additional risk is perceived, such as a deep wound or infection.
Concerningly, the author dealt with a fatal case of tetanus in a well-vaccinated horse, although, generally, vaccine breakdown would appear to be rare. Clinical cases of tetanus in horses are frequently fatal and no universally agreed evidence-based treatment protocol exists.
In addition to prevention of disease, administration of toxoid is also reported as part of the treatment protocol for clinical tetanus cases.
In a study of 61 cases of tetanus in horses, administration of toxoid in the face of the disease was one of very few treatments that appeared to improve the outcome (Reichman et al, 2008). Here, 43% of tetanus cases that received toxoid as part of their treatment survived versus 12% of those that did not (P=0.008). Interestingly, by comparison, administration of tetanus antitoxin was not associated with survival (P=0.578).
Equine viral arteritis is notifiable in the UK when suspected in stallions or in mares soon after breeding or AI.
The disease is common in continental Europe and the US. Thankfully, contagion from infected horses is generally short-lived, except in a proportion of stallions that can chronically shed virus in their semen.
As the virus survives well when chilled or frozen, it continues to pose a significant risk to AI mares. Thus, seropositive mares and geldings (and sexually immature colts) are only a risk to other horses if recently infected (in the past three to four weeks), whereas stallions should be regarded with greater and longer-term caution. Vaccination is, therefore, generally only used in stallions.
Interestingly, the Equip Artervac vaccine specifically claims “to reduce clinical signs and shedding of virus in nasal secretions after infection” and, therefore, strictly no product claims are made regarding protection against stallions becoming chronic shedders.
It is strongly recommended all stallions are established to be seronegative before vaccination is started to prevent future confusion about the cause of positive serologic blood tests.
Furthermore, a complete and compliant ongoing vaccination schedule is essential as, should the vaccine coverage lapse, confusion may again arise regarding whether seropositivity has resulted from vaccine or natural infection.
This is especially serious in breeding stallions and may necessitate highly costly establishment of freedom from persistent infection if seropositivity cannot be unequivocally attributable to vaccination.
EHVs are ubiquitous within equine populations and indeed establish latency in a high percentage (perhaps all) of exposed horses. This produces a rather interesting challenge to protect a horse against a virus it already has.
If a horse’s own natural immune responses cannot effectively destroy the virus, it is quite a high expectation that a simple adjuvanted killed virus vaccine (Equip EHV 1,4 or Bioquin H) could succeed.
EHV 4 is not a great health concern. Mild respiratory disease is recognised and also occasional abortions. Neurologic disease is unlikely.
In contrast, EHV 1 infection has greater capacity for causing abortion and/or neurologic disease depending on the exact strain. Vaccine efficacy claims are made for the protection of horses against EHV 1 or 4-related respiratory disease and abortion, but not neurologic disease.
Some concerns exist that vaccination may increase the risk of EHV 1 neurologic disease in horses. However, this is probably based on the confounded observation that the risk of EHV 1 myeloencephalitis is higher in older animals, and older animals are likely to have received more vaccine doses. Thus, EHV vaccination and EHV myeloencephalitis have not been proven to be causally related.
Where rotaviral diarrhoea exists as a problem or a particular concern in foals then advance planning allows vaccination of the late pregnant dam to establish good colostral (and subsequently milk) antibody levels that will be transferred to the neonatal foal.
Routine checks for good transfer of passive immunity to foals (circulating immunoglobulin G>8g/L) represents good general preventive medical practice, although should be mandatory alongside a rotavirus vaccine policy.
WNV infection may cause neurologic signs (such as tremors and ataxia) in a proportion of infected horses and mortality is significant. No known equine cases of WNV infection exist in the UK to date, although evidence exists of viral activity in avians in the UK and the presence of suitable vector mosquitoes that bite both birds and mammals.
Nevertheless, the immediate risk of disease is generally perceived by most as being too low to vaccinate routinely in the UK unless horses intend to travel internationally to WNV-endemic areas, such as southern/eastern Europe and the US, although this could change at any time. However, more cautious clients might request vaccination in anticipation of disease incursion into the UK in the future.
Where vaccines are used, it is sensible to time annual boosters prior to the expected season of highest fly activity. As a mosquito-vectored disease, WNV infection is most likely to occur during the summer/autumn and appropriate timing of vaccination should be considered in this context.