26 Nov 2024
Nicola Menzies-Gow discusses the importance of studies behind these conditions and the allocation of medicine.

Image © byrdyak/ Adobe Stock
Evidence-based medicine is a way of using the best scientific evidence to guide clinical decision-making and treatment recommendations. It starts with a clinical question and then the relevant scientific literature is reviewed to draw conclusions and make recommendations.
Scientific evidence includes scientific studies and opinions; however, not all evidence has the same strength. Recommendations from an expert are not as robust as the results of a well-conducted study, which is not as good as the results of a set of well-conducted studies. Therefore, in evidence-based medicine, the levels of evidence should be graded according to their relative strength and stronger evidence should be given more weight when making clinical decisions.
Three recent publications might influence clinical decision-making in the context of equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID) – namely the updated Equine Endocrinology Group (EEG) EMS and PPID guidelines, and the BEVA primary care guidelines for the diagnosis and management of PPID.
Finally, sodium glucose-like transporter 2 inhibitors (SGLT2i) are being used with increasing frequency in the management of equine insulin dysregulation and so a review of the relevant scientific literature is pertinent1.
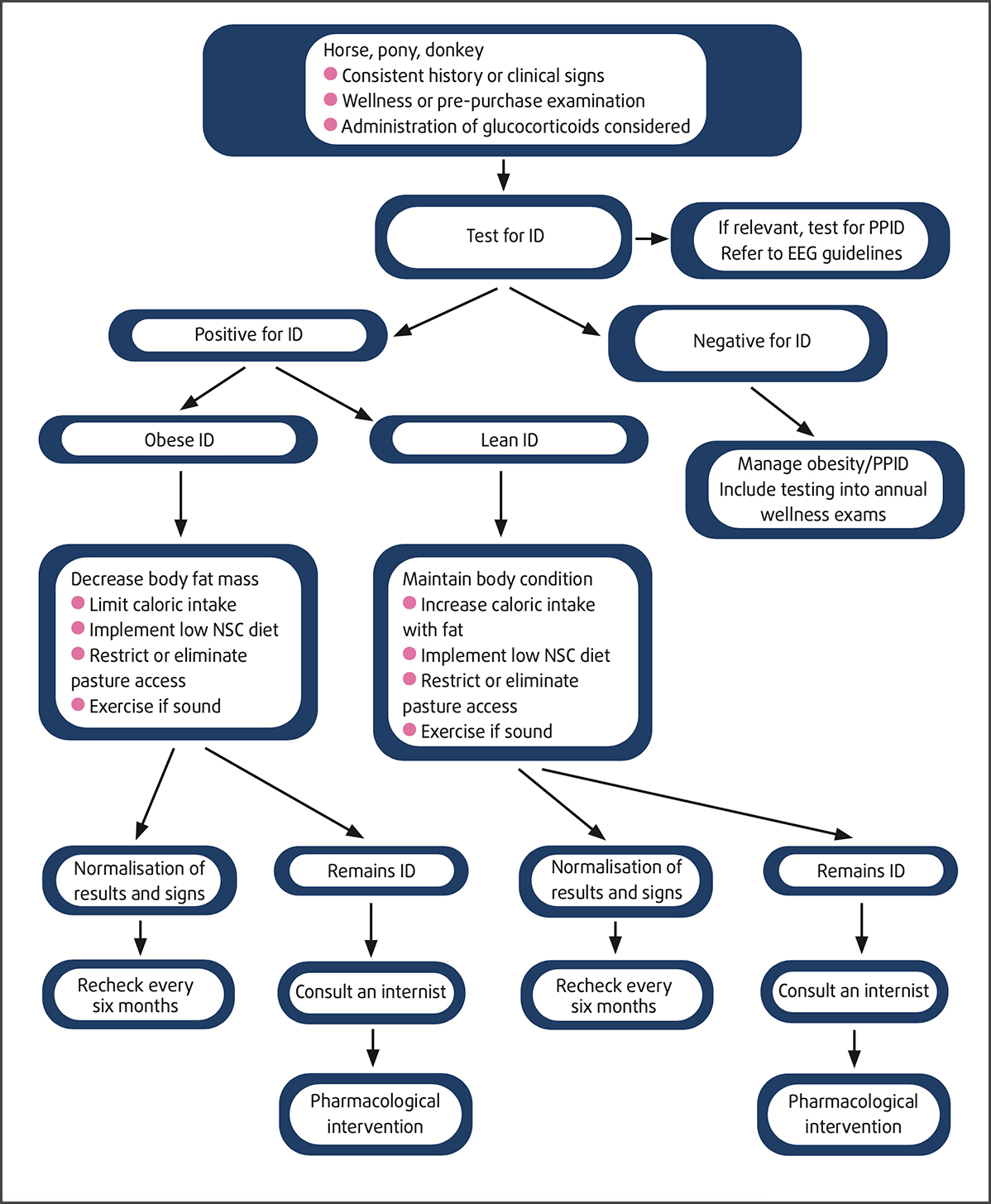
Algorithm for the diagnosis and management of insulin dysregulation.
The EEG is a group of clinicians and researchers that work together to advance our understanding of endocrine disorders in horses. The group contains key opinion leaders in the field who meet biennially to review diagnosis and treatment recommendations and discuss research.
The group provides recommendations for the diagnosis and treatment of several equine endocrine diseases including EMS and PPID. These recommendations were updated in 2024 and 2023 respectively, and are freely available online at equineendocrinologygroup.org
It should be remembered that these recommendations are based on expert opinion that is informed by scientific research rather than reviews of the scientific literature.
Hoof care is essential in all cases. Laminitis can occur without inducing easily detectable lameness, and radiographs are recommended to identify structural changes. In at-risk cases, regular care every 4 weeks by an experienced farrier is highly recommended.
Any exercise is good unless laminitis is present. All levels of exercise are beneficial for accelerating weight loss in obese animals and improving insulin sensitivity.
Drugs described below are currently being used off-label:
This class of drug is to be used when horses are affected by hyperinsulinaemia-associated laminitis (HAL) and severe ID and are not responding to other measures. They can also be used as a first-line management strategy for confirmed acute HAL to rapidly decrease insulin concentrations. Those drugs should be used for a certain duration (three months); however, it is the experience of the group that some extreme cases might require longer treatments, even if managed properly with diet and foot care.
Pharmacokinetic data are missing for this class of drug, and currently used doses are 0.3mg/kg, PO, q24h for velagliflozin; 0.5mg/kg, PO, q24h for canagliflozin; and 0.05mg/kg, PO, q24h for ertugliflozin and empagliflozin. In some cases, lower doses seem appropriate. Monitoring of postprandial insulin concentrations, hepatic function and triglyceride concentrations is strongly recommended. A transient increase in triglyceride concentrations is expected and usually not associated with hyperlipaemia. In some cases, however, marked hypertriglyceridaemia associated with clinical signs have been reported.
This drug is to be used for cases with weight loss resistance (no documented response after a minimum of 30 days on weight loss diet, with or without exercise) or for accelerated management of obesity. Levothyroxine is to be administered at 0.1mg/kg, PO, q24h (48 mg or 4 teaspoons of the powdered product for a 500-kg horse) while also controlling caloric intake. Weight loss is usually achieved after 3 to 6 months of therapy. At that time, treatment can be gradually reduced and discontinued.
Refer to EEG guidelines. PPID is an exacerbating factor for ID speculated to be a consequence of hormone products such as corticotropin-like intermediate peptide secreted from the pars intermedia.
Regular monitoring of ID cases is recommended, and methods include measuring insulin concentrations while the horse is on its current diet (hay or hay and controlled pasture access). As feeds are changed, postprandial insulin concentrations provide useful information on the individual horse’s response to their new diet and, indirectly, the risk of laminitis.
Pasture grass represents a source of sugars and amino acids that varies over time and season, depending on temperature, sunlight, rainfall, and use of fertilisers, and it is useful to assess the individual horse’s response to this component of their diet before easing restrictions on grazing. It is noted that insulin concentrations are affected by season, with higher concentrations detected in winter, suggesting a winter-associated exacerbation of ID. Accordingly, care should be taken to avoid overfeeding or adding high-NSC feeds in the winter months.
As age and PPID are factors that can exacerbate ID, it is recommended to reassess horses as they grow older using postprandial insulin concentrations and PPID testing (above 12 years). Close monitoring for early signs of laminitis is recommended, and the modified-Obel scoring system is recommended to better assess horses with HAL.
The development of clinical guidelines is standard practice in human health care, and these have been shown to influence decision-making in clinical settings.
BEVA initiated the development of guidelines for clinical practice aimed at equine primary care in an ambulatory setting.
The guidelines are developed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework, which involves identifying questions relevant to clinical practice, appraisal of the current veterinary evidence for each question and making recommendations based on the available evidence.
For the PPID guidelines2, the questions were categorised into four areas:
The final two areas are relevant to the management of PPID. The results developed into recommendations:
Pharmacologic treatments and other treatment/management options include the following:
Regarding the monitoring of pergolide-treated cases:
Review of the scientific literature related to use of SGLT2i in horses – SGLT2i are a novel class of oral hypoglycaemic agents used in combination with lifestyle changes in the management of human metabolic syndrome that has many similarities with EMS.
SGLT2 receptors are responsible for 90% of the renal glucose reabsorption that occurs in the proximal convoluted tubule. Therefore, these drugs increase urinary glucose excretion by suppressing glucose reabsorption from the glomerular filtrate, resulting in urinary calorie loss with consequent weight loss and improvements in insulin dysregulation (ID), hyperglycaemia, hypoadiponectinaemia and hyperleptinaemia.
No licensed veterinary drugs are available for treating ID and preventing insulin-associated laminitis in horses. Therefore, the use of SGLT2i for the control of equine hyperinsulinaemia with the goal of improving recovery from associated active laminitis or preventing future laminitis has recently been advocated.
A small number of published studies report the use of the SGLT2i canagliflozin3-5, ertugliflozin6,7 and velagliflozin8,9 to aid the management of equine ID.
However, the doses used are largely extrapolated from human studies, with limited consideration of species-specific variations.
In addition, limited evaluation exists of the fundamental differences between ID in horses and humans – particularly the fact that most horses with ID remain hyperinsulinaemic, but normoglycaemic, such that increased urinary loss of glucose may not explain the beneficial effects of these drugs.
Further study of the potential deleterious effects of treatment-associated hypertriglyceridaemia is required, together with the effect of SGLT2i therapy on circulating concentrations of adipokines in horses.


Treatment and diet and exercise recommendations.