13 Feb 2017
David Rendle discusses the risk factors and methods of prevention for this condition, which mainly affects performance horses.
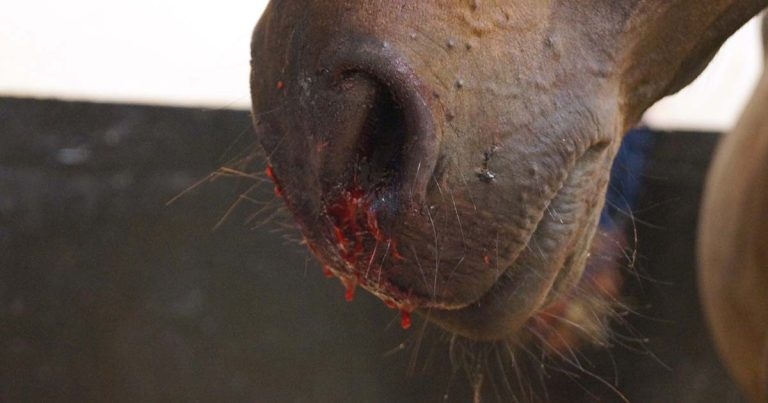
Figure 1. Epistaxis is a sign of severe exercise-induced pulmonary haemorrhage, but the majority of cases go unnoticed unless endoscopy is performed following racing.
Exercise-induced pulmonary haemorrhage (EIPH) is a performance-limiting condition that has a huge financial impact on the equine industry.
It is predominantly a disease of racehorses, but may affect horses involved in other high-intensity pursuits, such as polo and eventing, and, occasionally, other disciplines, such as showjumping and dressage. In addition to financial losses, implications for animal welfare exist as the disease can result in permanent pulmonary pathology and, rarely, sudden death.
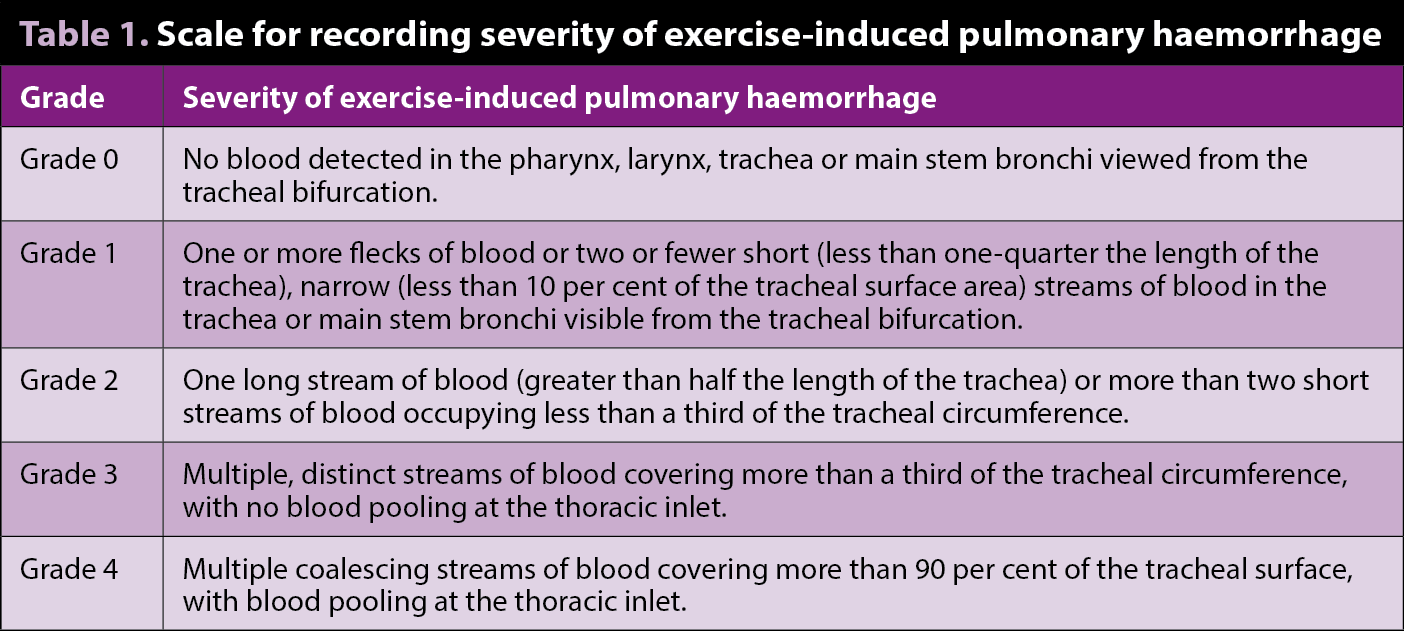
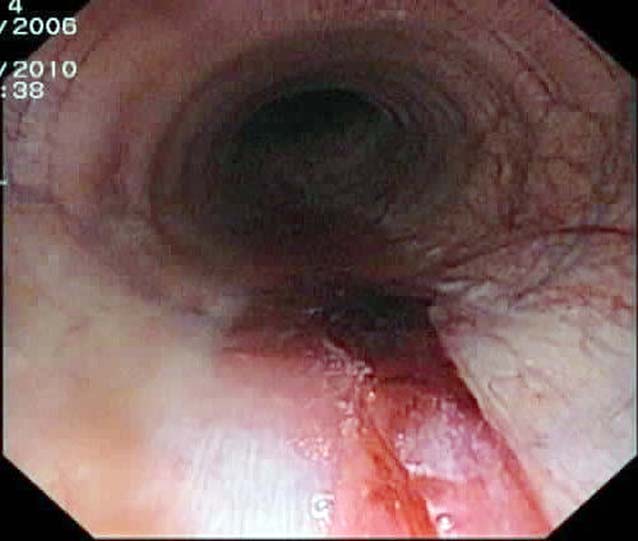
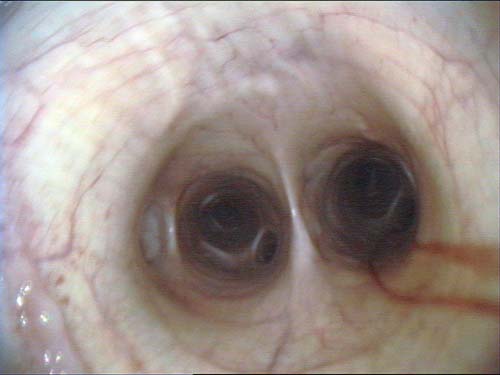
The clinical signs of EIPH may include poor performance, stopping during exercise, epistaxis (Figure 1) and sudden death during exercise. EIPH is most often defined as the presence of visible blood in the trachea on endoscopic examination (Table 1, Figures 2 and 3)1. However, some horses that have no visible blood in the airways on endoscopy will have marked haemorrhage in the more distal airways sufficient to cause red discolouration of tracheal wash (Figure 4) or bronchoalveolar lavage fluid (BALF; Figure 5).
EIPH occurs as a consequence of stress failure of the pulmonary capillaries. This may be the result of increased positive blood pressure in the capillary, increased negative pressure in the alveoli or a combination of both.
Pulmonary arterial and venous blood pressures will increase three-fold to four-fold during strenuous exercise as a result of increased cardiac output frequently to levels that exceed those that cause capillary failure in vitro2. Pressures are highest in the caudodorsal lung lobes where EIPH typically occurs.
A further factor that may increase the risk of EIPH is veno-occlusive remodeling, which may occur as a result of exercise or repeated haemorrhage and is most pronounced in the caudodorsal lung lobes. Increased collagen deposition and smooth muscle hypertrophy occur in the small diameter veins, increasing their strength and stiffness, but at a cost of reduced diameter and, hence, increased resistance to blood flow3,4. This resistance may further increase capillary pressures and increase the risk of capillary failure and subsequent haemorrhage.
A number of investigations of risk factors for EIPH have been performed in both Thoroughbreds and standardbreds. Some risk factors identified vary between different racing jurisdictions, but, in all studies, the risk of EIPH increases with increasing intensity and speed of exercise2. Bleeding is far more likely to occur in the race itself than during training5,6.
The risk of EIPH increases with career length. Hurdlers or steeplechasers, which tend to be older and have longer careers in racing, are more likely to suffer from EIPH than horses racing on the flat7,8. Horses that have been racing for more than two years are at higher risk9 and those with at least 50 race starts were 1.78 times more likely to have endoscopically evident EIPH, according to a study10.
The association between sex and risk of EIPH is unclear. Although the disease occurs more commonly in males, this may be confounded by racing factors as mares and fillies are more likely to be retired to stud at a younger age10,11.
Age appears to be a risk factor for EIPH, but this observation is inevitably confounded by the time spent in racing and the number of races run. These factors will be more relevant to risk than age itself2.
Diastolic pressures in the left side of the heart will have a major influence on pulmonary venous and, hence, pulmonary capillary pressures. Cardiac dysfunction may, therefore, contribute to the development of EIPH.
Paroxysmal atrial fibrillation is the cardiac abnormality most commonly associated with EIPH2.
Exercise not only increases capillary pressure, but can increase the negative pressure within the alveoli 10-fold12. Any obstruction of the upper airway results in a further, potentially marked, increase in negative pressure13, suggesting static and dynamic causes of upper respiratory tract dysfunction could be potential factors in the development of EIPH.
However, horses with recurrent laryngeal paralysis in Hong Kong were no more likely to develop EIPH than matched cohorts prior to surgery; after surgery they were more likely to develop epistaxis14.
Airway inflammation has the potential to cause airway narrowing, resulting in more negative alveolar pressures and a greater risk of capillary failure.
When haemorrhage occurs, blood constituents, which are proinflammatory, may cause a spiral of increasing inflammation and haemorrhage. Instillation of blood into the airways causes a mild inflammatory response for up to two weeks18 and, when blood enters the interstitium, it has the potential to promote the development of the fibrotic lesions that typify EIPH.
No evidence exists that clotting disorders contribute to the development of EIPH21.
While it has not been the subject of study, rest from racing is likely to be the most effective treatment, albeit an unpopular one. Rest will prevent further bleeding and hemosiderin accumulation, and the associated inflammation, fibrosis and remodeling.
Furosemide has been used widely in the treatment of EIPH for more than 40 years, despite its definitive mechanism of action in EIPH being unknown.
Furosemide is a loop diuretic that reduces intravascular volume and limits the increase in right atrial – as well as pulmonary arterial, venous and capillary – pressures. Furosemide also has direct effects in the lungs via smooth muscle dilation of both pulmonary veins (reducing capillary pressure increases) and bronchi (reducing negative pressure changes in the alveoli).
A total of 167 horses were raced twice over the same distance, one week apart. Each horse received 500mg furosemide by IV injection four hours before one race and a saline placebo four hours before the other.
After receiving furosemide, horses were less likely to bleed and less likely to bleed severely. Two-thirds (81 out of 120) of horses that bled after receiving saline bled by at least a grade less after receiving furosemide.
In the UK, furosemide cannot be used prior to racing and can only be used to reduce the incidence of EIPH during training. The optimal dose and timing of furosemide in relation to exercise have not been determined, but doses of 0.5mg/kg (“low dose”) to 1mg/kg (“high dose”) are typically used two to four hours before exercise. Water deprivation is sometimes practised in association with the use of furosemide, but cannot be condoned.
Vasodilatory drugs, including phosphodiesterase inhibitors (such as pentoxifylline or sildenafil), angiotensin-converting enzyme inhibitors (such as enalapril), alpha-2-adrenergic agonists (such as clonidine) and nitric oxide analogues, have not been demonstrated to be effective in reducing the development of EIPH24-26.
Drugs such as aminocaproic acid, oestrogens, aspirin, bioflavonoids and vitamin C, which inhibit fibrinolysis and, therefore, promote clot formation, are ineffective27,28 as clotting disorders are not a feature of EIPH.

External nasal strips are designed to limit the collapse of the upper airways, increasing air flow and limiting the development of negative pressure in the terminal airways. Evidence pertaining to their effects is mixed and further higher quality field trials are needed.
Some investigations of their use have reported a reduction in the number of erythrocytes in BALF, while others have failed to identify a difference in endoscopic findings29-31. Nasal strips are not permitted under the rules of racing, but are permitted under Fédération Equestre Internationale rules.
Lower airway inflammation may be a factor in the development of EIPH, so its investigation and treatment is logical, but has not been evaluated critically. Cytological assessment of BALF provides a more sensitive and reliable means of assessing lower airway inflammation than cytology performed on tracheal aspirates, but is not popular in horses in training. If evidence of lower airway inflammation is identified on either tracheal wash or bronchoalveolar lavage (BAL) cytology or on the gross appearance of the airways then measures should be implemented to improve air hygiene and the use of glucocorticoids should be considered.
Between 1% and 4% of race starts will develop epistaxis2; however, 50% to 75% of Thoroughbred racehorses would have visible evidence of haemorrhage if endoscopy was performed two hours after racing23,32. If BAL was performed post-race, erythrocytes or haemosiderophages would be identified in virtually all racehorses33.
Pulmonary haemorrhage was judged to have contributed to death in 50 out of 143 racehorses in an international study of horses that were examined postmortem34 and 60% of racehorse deaths were attributed to pulmonary haemorrhage in a study in Australia35. However, cause and effect has not been demonstrated and will be difficult to demonstrate when the majority of horses exhibit a degree of pulmonary haemorrhage during racing.
As virtually all racehorses exhibit pulmonary haemorrhage, the severity of haemorrhage needs to be considered when determining its significance. Unfortunately, this tends to be a calculated guess. Early investigations of EIPH and performance were low powered, had a variable quality of study design and produced conflicting results.
Two more robust prospective studies have been performed in Victoria, Australia32, and South Africa36, looking at 744 and 1,000 Thoroughbred flat racehorses, respectively.
In the Australian study, horses with EIPH of grade two or greater were four times less likely to win and two times less likely to finish in the first three; they also finished significantly further behind the winner than horses that had not bled. Horses categorised as grade 0 or 1 were three times more likely to be in the top 10% for earnings. In South Africa, horses without EIPH were twice as likely to win, finished an average of one length ahead of horses with EIPH, and were twice as likely to be in the highest 10% for race earnings.
In the Australian study, no association existed between a single episode of EIPH of less than or equal to grade three and earnings, total number of starts, wins or placings achieved during a horse’s career. However, horses with severe (grade four) EIPH had shorter career duration, lower earnings and fewer starts than horses not diagnosed with EIPH. Epistaxis, another indicator of severe EIPH, has been associated with lower career earnings in Thoroughbreds in the UK37.
EIPH is associated with poor performance, prematurely terminates racing careers and presents a huge cost to the racing industry. The majority of risk factors relate to the intensity and duration of exercise. Other conditions that may contribute to the development of EIPH – such as cardiac arrhythmias, dynamic collapse of the upper airway and lower airway inflammation – should be investigated and treated or managed where possible.
Rest from high-intensity exercise is likely to reduce the risk of recurrence and progression. Air hygiene and treatment of lower airway inflammation may help at least a subsection of cases. Furosemide is an effective preventive measure in some horses, but cannot be used prior to racing in the UK. No evidence exists to demonstrate pharmaceutical or nutraceutical alternatives to furosemide are effective.