25 Sept 2017
Aimi Duff highlights the main disease threats to the UK equine population, their transmission and clinical symptoms.

IMAGE: The Donkey Sanctuary Kenya.
The trade of horses across the globe has grown exponentially over the past decade (Herholz et al, 2008).
Additionally, internationally competing and breeding horses cover great distances to get to events and studs, and equine biological products, such as plasma, vaccines, semen and embryos, are sold globally (Herholz et al, 2008). This movement, coupled with the effects of climate change on vector-borne disease, increases the threat posed to the equid population of the UK.
This article summarises the characteristic features of the main disease threats.

African horse sickness (AHS) is an insect-borne viral disease of horses, which is endemic in sub-Saharan Africa (Herholz et al, 2008; Mellor and Hamblin, 2004). The AHS virus (AHSV) has nine serotypes; however, outbreaks beyond Africa are invariably associated with AHSV-9 (Herholz et al, 2008; MacLachlan and Guthrie, 2010; Mellor and Hamblin, 2004; Wilson et al, 2009).
Following viral transmission, viral replication within regional lymph nodes occurs, resulting in secondary viraemia and dissemination of virus to target tissues – typically the spleen, lymphoid tissue and lungs, which amplifies the viraemia (Mellor and Hamblin, 2004). This widespread dissemination is associated with the development of one or a combination of clinical presentations; namely pulmonary (peracute), cardiopulmonary (mixed), cardiac (subacute) and/or AHS fever (Table 1; Figures 1 and 2; Herholz et al, 2008; Mellor and Hamblin, 2004).
In susceptible equid populations, the consequences of infection can be devastating, with up to 90% mortality reported (Lo Lacono et al, 2013; MacLachlan and Guthrie, 2010; Mellor and Hamblin, 2004; Wilson et al, 2009).
Culicoides imicola, and to a lesser extent Culicoides obsoletus and Culicoides pulicaris, act as the vectors for AHSV transmission (Mellor and Hamblin, 2004; Wilson et al, 2009). These species have been identified across southern Europe, along with horses infected with the virus (Mellor and Hamblin, 2004; Wilson et al, 2009).
It has been speculated climate change has encouraged the spread of C imicola northwards, resulting in disease outbreaks beyond Africa and into Spain, Portugal, Italy, Greece and Switzerland (Herholz et al, 2008; Lo Lacono et al, 2013; MacLachlan and Guthrie, 2010; Mellor and Hamblin, 2004). It has also been suggested the spread of C imicola in Europe has not changed, but rather they are being identified more frequently as a result of surveillance for bluetongue virus, which is carried by the same vector.
Increased ambient temperature is associated with increased rates of infection with AHSV and viral replication (Lo Lacono et al, 2013; Mellor and Hamblin, 2004). Viral infection of vectors can also remain latent at temperatures under 15°C, with replication and increased probability of transmission occurring as temperature subsequently increases (Mellor and Hamblin, 2004). Latency may enable AHSV to overwinter in Morocco and Spain (Mellor and Hamblin, 2004), and potentially other European countries it spreads to. There would be potential for a similar situation to develop in the UK, should AHSV be introduced.
With sporadic AHS infections having occurred in the EU, and the fact a viable vector population is present, a credible threat exists of AHS being introduced to the UK (Defra, 2016). The outbreak of bluetongue virus in the UK, which has been attributed to C obsoletus and C pulicaris transmission, also indicates the potential for spread of disease carried by these vectors.
West Nile virus (WNV) is an arbovirus maintained in the environment in birds and transmitted by mosquitoes, in which the virus survives within salivary glands (Castillo-Olivares and Wood, 2004; Chapman et al, 2016; Herholz et al, 2008). However, vertebrates such as horses and humans become infected if they are bitten by infected mosquitoes (Castillo-Olivares and Wood, 2004; Herholz et al, 2008).

Following viral inoculation, viral replication within the host tissues and lymph nodes results in viral transport by the lymphatic system to the blood stream, and subsequent viraemia (Castillo-Olivares and Wood, 2004). Vector feeding during this time may allow further disease transmission (Castillo-Olivares and Wood, 2004).
WNV was first described in Uganda in 1937, but more outbreaks have been reported in humans and horses in Morocco, Romania, Tunisia, Russia, Italy, the US, Israel and France, with devastating effects seen in susceptible populations (Buckley et al, 2003; Castillo-Olivares and Wood, 2004; Chapman et al, 2016; Herholz et al, 2008).
In the past 20 years, WNV has been responsible for frequent disease outbreaks and cases of severe encephalomyelitis attributable to an evolved WNV variant, which is more readily transmitted in population-dense areas (Buckley et al, 2003; Castillo-Olivares and Wood, 2004; Herholz et al, 2008).
WNV infection does not always result in signs of clinical disease (Castillo-Olivares and Wood, 2004). In outbreaks, approximately 10% of equine and 1% of human cases have displayed pyrexia and neurological signs associated with polioencephalomyelitis of the spinal cord, mesencephalon and rhombencephalon (Castillo-Olivares and Wood, 2004).
Clinical signs associated with infection of these structures include ataxia, single or multiple limb paresis or paralysis, skin and muscle fasciculations, or muscle tetany. Damage to the medulla oblongata, thalamus, pons and reticular formation have also been reported, causing altered mentation, dysmetria, hyperaesthesia and cranial nerve deficits. Sudden death can occur and euthanasia is frequently indicated.
The outcome of WNV infection depends on the viral strain involved and efficiency of the host’s innate immune response. It is not known whether latent infection can persist in the horse (Castillo-Olivares and Wood, 2004).
Viral replication can occur in vector salivary glands, but this is dependent on environmental temperature and humidity (Castillo-Olivares and Wood, 2004; Chapman et al, 2016). Therefore, in temperate climates, disease outbreaks are typically only seen during late summer and autumn (Castillo-Olivares and Wood, 2004; Herholz et al, 2008). Climate change not only promotes viral replication and vector transmission, but may also increase the geographical distribution of mosquitoes and enable the virus to survive over winter in vector species (Chapman et al, 2016).
Serological investigations of resident and migrant British birds have identified circulating WNV in the UK, possibly as a consequence of vector-borne transmission from African migrant birds to resident populations (Buckley et al, 2003; Castillo-Olivares and Wood, 2004; Herholz et al, 2008). An estimated 34 species of mosquito exist in the UK, several of which are capable of carrying and transmitting arboviruses (Chapman et al, 2016).
One of the most abundant UK mosquito species is thought to be Culiseta annulata, which is known to bite both birds and mammals, and is capable of WNV transmission (Chapman et al, 2016). Further work is, however, necessary to establish vector-competence in the UK (Chapman et al, 2016).
Worldwide, a number of arboviruses can cause disease in horses and are of concern for their zoonotic potential (Chapman et al, 2016; Weaver et al, 2004):
Arboviruses are responsible for causing rapidly progressive encephalitis or encephalomyelitis, which manifests as ataxia, paresis, paralysis, pyrexia, inappetence, hypersensitivity to touch and sound, hyperexcitability, blindness, muscle fasciculations and depression (Chapman et al, 2016; Herholz et al, 2008).
Some of these viruses (JEV, EEEV, WEEV and VEEV) have been identified in mosquito populations in Europe, in species that feed on mammals (Balenghien et al, 2008; Börstler et al, 2016; Chapman et al, 2016; Herholz et al, 2008; Schönenberger et al, 2016). Evidence is also available from avian serology surveys of arboviral circulation in Europe, although these have not been associated with disease outbreaks (Gould et al, 2006).
In Australia, KUNV and MVEV cause sporadic disease outbreaks. VEEV was first isolated from a horse in South America in the 1930s, with outbreaks being reported in Columbia, Ecuador and Peru, as well as Venezuela and, more recently, in central America and the US (Weaver and Barrett, 2004). JEV has been responsible for disease outbreaks across Asia (Weaver and Barrett, 2004). While these viruses have not been identified in the UK, there is potential for their introduction within vectors travelling with animals, plants, people or equipment (Gould et al, 2006). The risk of a disease outbreak is low because UK environmental conditions do not favour amplification of these viruses within arthropods, nor transmission (Gould et al, 2006).
Equine encephalosis (EE) is a Culicoides-transmitted orbivirus, which is endemic in the temperate regions of Africa, where 50% to 60% of horses, donkeys and zebras have tested seropositive (Wohlfender, 2009; MacLachlan and Guthrie, 2010).
The virus has a similar epidemiology to AHS, but with a higher transmission rate (Wohlfender, 2009; MacLachlan and Guthrie, 2010). Disease is typically mild and fatal disease is rare. Clinical signs include pyrexia, inappetence, and oedema of the face and limbs. Mucous membranes may appear brown due to mild icterus and congestion, and patients may appear ataxic, depressed or hyperexcitable (Wohlfender, 2009).
Outside Africa, disease is rare, but outbreaks have been reported in Israel, North America and southern Europe (Castillo-Olivares and Wood, 2004; MacLachlan and Guthrie, 2010).
The threat to the UK is low, but introduction is possible through importation of infected animals from an endemic area and transmission by native Culicoides species (Wohlfender, 2009).
Equine infectious anaemia (EIA) is a notifiable disease of horses caused by a lentivirus, which is able to persist in equine leukocytes for life (Defra, 2010; Herholz et al, 2008).
Transmission occurs in body fluids through contact with saliva, milk, blood or blood products, or other body secretions (Defra, 2010; Herholz et al, 2008). Mechanical transmission by blood-feeding biting insects during feeding is a common means of transmission of infectious blood between horses, with flies in the tabanidae family commonly responsible; for example, horse flies, stable flies and deer flies (Defra, 2010; Herholz et al, 2008). Iatrogenic transmission may occur through contaminated equipment and, particularly, needles (Herholz et al, 2008).
Acute EIA infection is characterised by pyrexia and haemolytic anaemia (Herholz et al, 2008) and there may also be weakness, limb and ventral oedema, lingual petechial haemorrhages and cardiac dysrhythmias (Figure 2). Sudden death can also occur (Herholz et al, 2008).
EIA is endemic in South America, most of Africa, Romania and Italy, with sporadic outbreaks having been reported in many other European countries (Defra, 2014a; Herholz et al, 2008). In 2006, an outbreak occurred in Ireland, in which 38 cases were identified in the six months following initial diagnosis in one mare (Brangan et al, 2008; More et al, 2008). The cause of the Irish outbreak was attributed to the importation of plasma from Italy (More et al, 2008). Iatrogenic and vector-borne transmission led to the rapid dissemination of disease (More et al, 2008).
The disease has been identified in imported horses in the UK, but an outbreak of disease has not occurred since 2010 (Defra, 2014a).
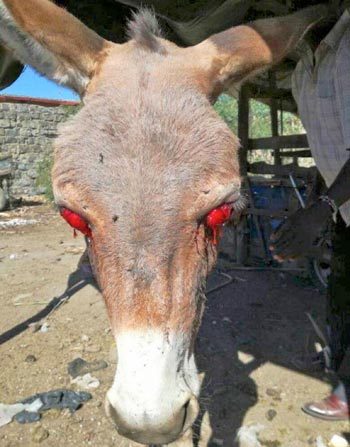
Equine viral arteritis (EVA) is caused by an arterivirus. Clinical signs include pyrexia, abortion, periorbital/scrotal/limb/mammary oedema, conjunctivitis, nasal/ocular discharges, depression and urticaria (Holyoak et al, 2008; Wood et al, 1995).
Following infection, viraemia may be present for up to 20 days, during which the virus may disseminate to other horses via secretions, including saliva, urine, milk, semen, nasal discharge and faeces (Holyoak et al, 2008; Wood et al, 1995).
Stallions may remain persistently infected through accessory gland infection, which can result in viral transmission to mares covered both naturally and through artificial insemination (Holyoak et al, 2008; Wood et al, 1995).
The first outbreak of EVA in the UK occurred in 1993, in a mare that had been naturally covered by a stallion imported from eastern Europe (Wood et al, 1995). During this outbreak, EVA spread directly through respiratory secretions of acutely infected mares and the use of chilled semen from the affected stallion (Wood et al, 1995). More outbreaks in France and the US have occurred following the insemination of infected semen (Holyoak et al, 2008).
To prevent further outbreaks in the UK, stallions should be tested and confirmed free from infection, as recommended by the Horserace Betting Levy Board Code of Practice (HBLB, 2017; Wood et al, 1995).
Equine piroplasmosis (EP) is a tick-borne protozoal infection affecting horses, donkeys, mules and zebras.
The protozoa, Babesia caballi and Theileria equi infect erythrocytes and multiply, resulting in erythrolysis (Carson, 2013; World Organisation for Animal Health [OIE], 2017). Clinical signs include anaemia, jaundice, pyrexia, inappetence, dyspnoea, depression, haematuria, petechial haemorrhages, lethargy, limb swelling and, potentially, sudden death (Carson, 2013; de Waal, 1992; OIE, 2017). The incubation period for infection can be up to 30 days, with some animals remaining as subclinical carriers. A carrier state may persist for life, with disease recrudescence occurring at times of stress or immune suppression (Carson, 2013).
Twelve tick species have been implicated as vectors; however, most of these are only present in tropical and subtropical climates (Carson, 2013). Tick feeding on an infected horse results in protozoal ingestion and, potentially, transmission on to a susceptible horse during subsequent feeding (Carson, 2013; OIE, 2017).
Babesia can be transmitted transovarially in ticks, resulting in amplification of infection and transmission through infected tick larvae; however, Theileria can only be transmitted via infected tick salivary gland secretions (Carson, 2013; OIE, 2017). Iatrogenic transmission through contaminated blood products and equipment, and transplacental infection, are also possible (Carson, 2013; OIE, 2017).
Infection is endemic in several countries where vectors are prevalent, including countries in central and South America, Cuba, the Caribbean, Asia, Africa and southern Europe (Carson, 2013; OIE, 2017).
Movement of horses with subclinical infection from these regions presents a constant threat to UK equid populations (Carson, 2013). This is highlighted by the outbreak of EP, which occurred in Ireland in 2009 (Carson, 2013). No requirement exists for horses to be treated with acaricides prior to importation to the UK. Tick species present in the UK are capable of vector transmission should the disease be introduced.
The threat of EP in the UK can be reduced by recommending acaricide treatment and performing serology to determine exposure prior to importing horses from areas of endemic infection (Carson, 2013).
Several of these emerging disease threats are “notifiable”, meaning vets are legally obliged to report confirmed, and even suspected, cases to their nearest AHPA office (Defra, 2014b). Movement restrictions should be imposed, with control zones around the premises introduced (Defra, 2014b). The AHPA can advise on the area of these zones where appropriate, as these will depend on the pathogen and transmission risk (Defra, 2014b). Biosecurity protocols should be implemented. These restrictions should be left in place until investigations by AHPA vets are complete and freedom from disease confirmed (Defra, 2014b).
The following are considered notifiable diseases of horses in the UK (Defra, 2014c):
Credible threats exist to the UK equid population from a number of exotic diseases. Vigilance and an open mind are, therefore, necessary to ensure rapid detection and containment of infected animals.