30 Jul 2018
Sarah Smith and Victoria Savage discuss how to diagnose these abnormalities, and suggests various treatment and prevention options.
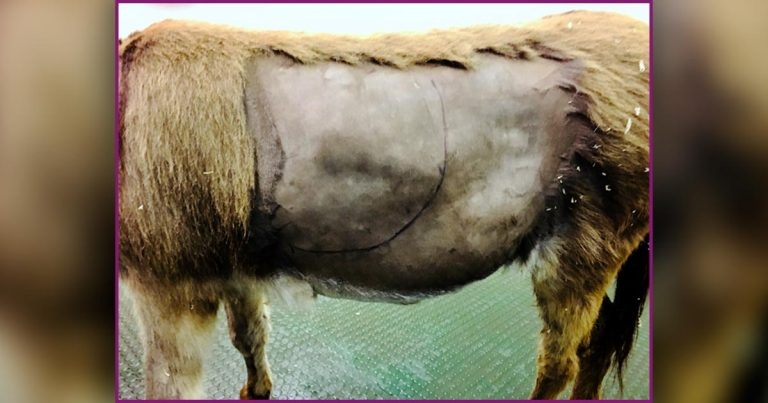
A donkey with a gastric impaction. See Figure 3.
Gastric abnormalities in the horse often present with non-specific and variable clinical signs, including poor performance, inappetence, lethargy and colic.
Historically, diagnosis was problematic; however, improvements in gastroscopy and ultrasonography have facilitated affordable, readily available visualisation of the stomach and characterisation of gastric disorders, including gastric ulceration, impaction and neoplasia. The aetiology, diagnosis and management of these conditions are discussed in depth.
The term equine gastric ulcer syndrome (EGUS) has been used for the past 20 years as an all-encompassing name for both squamous and glandular ulceration in the equine stomach. However, it is important these two conditions are considered as separate entities, as it is likely they have both a different aetiology and different response to treatment.
The aetiology of equine squamous gastric disease (ESGD) and response to treatment is well described. Good evidence exists that this condition results from the exposure of the squamous mucosa to acidic stomach contents, which it is not designed to resist. This occurs due to factors that increase the acidity of the stomach contents and exposure of the squamous mucosa to the stomach contents (Figure 1).
The prevalence of ESGD is known to increase with certain management-associated factors, such as increasing exercise intensity, dietary changes (increased starch/grain intake, more time between forage feeds, feeding straw as the only forage type and intermittent access to water) and environmental stressors (such as lack of direct access to other horses).
The highest prevalence of ESGD is found in Thoroughbred racehorses in training, which are exposed to many risk factors, and the prevalence reduces to a low level in pleasure horses that rarely leave their home environment.
The prevalence of equine glandular gastric disease (EGGD) varies from approximately 15% up to more than 60%. However, a prevalence of 50% to 60% has been found in both pleasure and competition horses, without an obvious association with the risk factors known to predispose to ESGD. Unlike the gastric squamous mucosa, the glandular mucosa is adapted to withstand the highly acidic contents of the ventral stomach – and, therefore, it is unlikely acidity is the sole cause of ulceration in this region.
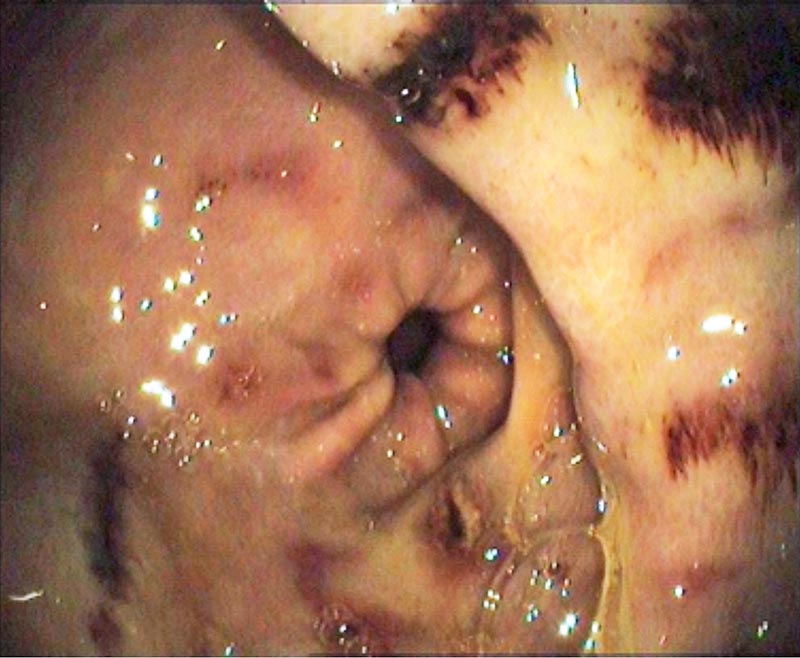
It is thought EGGD is likely to be due to a breakdown in the protective mechanisms of the glandular mucosa (Figure 2). In humans, the primary contributing factors are the presence of the bacteria Helicobacter pylori and the use of NSAIDs. Although research into EGGD in horses has focused on these factors, limited evidence exists to support their role, with little evidence for the involvement of Helicobacter, and no effect of clinical doses of phenylbutazone and suxibuzone on EGGD after 15 days’ treatment (Andrews et al, 2009).
Very few studies are available investigating an association between EGUS and equine performance. Poor body condition and rough hair coat is associated with a higher prevalence of gastric ulceration in Thoroughbred racehorses.
In both Thoroughbred and standardbred racehorses, the presence of EGUS is associated with reduced performance; however, this is unlikely to be transferable to horses in other disciplines where performance is not directly related to speed. It is generally perceived horses displaying stereotypic behaviour or with alterations in behaviour are more likely to have EGUS; however, limited data exists to support it.
Associated risk factors, such as reduced appetite, may reduce performance; however, the impact of EGUS and associated gastric pain on performance in sports horses and pleasure horses is not well understood. For this reason, in many clinical situations it is necessary to treat the gastric ulceration and assess any changes in the horse’s behaviour in response to treatment.
Although some evidence supports a relationship between the severity of ESGD and the development of clinical signs, clinical impression in many cases ignore this trend; both horses with severe ulceration that do not show clinical signs and horses with relatively mild lesions and apparent clinical signs that improve following treatment and resolution of the ulceration.
The European College of Equine Internal Medicine Consensus Statement on EGUS coined the phrase “no acid, no ulcer”, implying all EGUS requires treatment with acid-suppressing medication and this is indeed the cornerstone of EGUS treatment (Sykes et al, 2015). This is most commonly administered as H2-receptor antagonists (proton pump inhibitors) available in a variety of oral paste formulations.
The products licensed for use in the UK are available as omeprazole in a buffered paste formulation, which is required to prevent degradation of the drug in the acidic environment of the stomach. A compounded formulation of omeprazole has become available for IM injection to be used under the cascade.
For the treatment of ESGD, buffered omeprazole paste given orally once daily at 4mg/kg for 28 days has been shown to cause resolution of ulceration in nearly 80% of cases, and 21 days treatment may well be sufficient. It has been suggested a lower dose, such as 2mg/kg, may be sufficient to resolve ESGD; however, this is likely to be influenced by factors such as gastric fill, which, in turn, influence absorption of the omeprazole paste. The recommendation from the ECEIM consensus statement is to treat with 4mg/kg buffered omeprazole paste once daily by mouth for 21 days.
Studies have shown the rate of healing of EGGD when treated with buffered oral omeprazole paste is lower, with only approximately 25% healed after 28 days. The reasons for this difference are poorly understood. However, it is interesting to note the similarity to human NSAID-induced ulceration, which takes 8 to 12 weeks of treatment with acid suppression to heal. In light of the suspected pathogenesis of EGGD, it seems logical to use mucosal protectants for the treatment of EGGD, in addition to omeprazole.
The best studied gastroprotectant available is sucralfate, which is known to improve mucosal healing by adhering to the mucosa, stimulating mucosal blood flow, increasing mucosal secretions and prostaglandin E production.
Prevention of ulceration is easiest for ESGD where the risk factors are well understood; prevention can primarily be affected by reducing the previously mentioned risk factors as much as possible.
In some cases, on-going acid suppression may be deemed beneficial when it is not possible to reduce the risk factors, there is repeated recurrence of ulceration or periods of time where the risk factors are markedly increased. When used for prevention, lower doses of oral omeprazole paste maybe trialled, often starting at 1mg/kg.
Prevention of EGGD is more challenging as the risk factors and causative agents are poorly understood. In some situations, ongoing acid suppression may be trialled; however, the justification for this is limited as equine study and human research has shown this to worsen ulceration.
Gastric impaction is a condition characterised by excessive accumulation of ingesta in the stomach. Primary gastric impaction is uncommon, and it is difficult to diagnose without exploratory laparotomy; however, impaction is common secondary to other gastrointestinal causes of colic and thought to occur due to alterations in motility.
A retrospective study included horses in which gastroscopy was performed as a part of a routine colic investigation and identified a 5.6% incidence of gastric impaction (Vainio et al, 2010). The following factors predispose to gastric impaction:
The clinical signs of gastric impaction are variable and often non-specific, including inappetence and mild colic. Dysphagia, decreased faecal output, mild pyrexia, excessive salivation and lethargy or acute, severe or recurrent colic are also reported. Clinical examination is often unremarkable. The most common laboratory findings include leukopaenia – a relative granulocytosis and hyperfibrinogenaemia. Hypoalbuminaemia and overall hypoproteinaemia are reported, and hypertriglyceridaemia and hyperbilirubinaemia can be observed, secondary to inappetence.
Diagnosis of a gastric impaction is by gastroscopy and can be made if the horse is starved for 16 hours, but a concretion of ingesta exists within the stomach that precludes visualisation of the margo plicatus. If no other gastrointestinal abnormalities are identified, the impaction is considered primary. Transabdominal ultrasound examination may aid in assessment of the severity of gastric distension (Figure 3).
Management of gastric impaction includes:
Short-term prognosis is favourable, with 90% survival to discharge with medical management. The condition recurred in 11% of horses, of which 50% were euthanised due to recurrence and 50% were treated successfully for a second time (Vainio et al, 2010).
A severe form of gastric impaction of unknown aetiology has been reported in Europe, whereby the stomach becomes gradually impacted with large volumes (up to 122kg) of material. Affected horses have severe gastric distension, which may be palpable rectally, before showing clinical signs or poor performance. Accompanying changes, including gastric ulceration and adhesions to the diaphragm, usually prompt investigation and subsequent diagnosis. The prognosis for long-term survival in these cases is likely to be guarded.
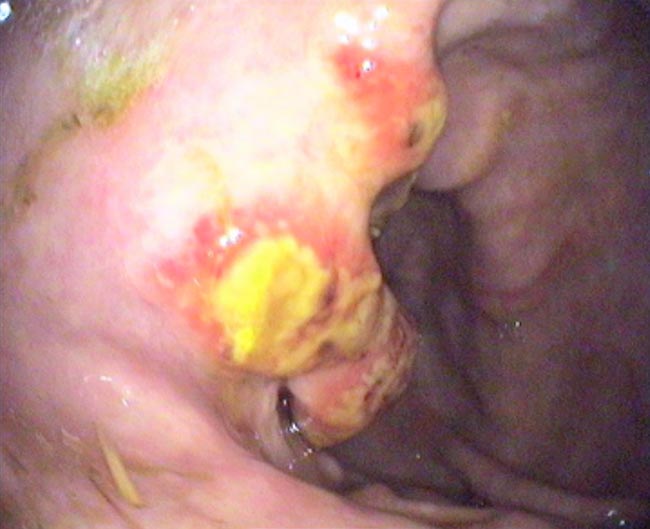
Equine gastric neoplasia is a rare condition with a poor prognosis for short-term survival. The most commonly seen gastric neoplasm is squamous cell carcinoma (Taylor et al, 2009). These cases tend to present very late in the disease course; the most common clinical signs are inappetence, weight loss, lethargy, colic and fever.
Routine colic investigation tends to localise the lesion, which can be confirmed with gastroscopy, most commonly identifying a single, ulcerated, necrotic lesion in the glandular portion of the stomach. The average time from onset of clinical signs to death or euthanasia is four weeks, and, in many cases, metastatic lesions were identified at postmortem. Other previously reported gastric neoplasms include leiomyoma, lymphoma, adenocarcinoma and mesothelioma.
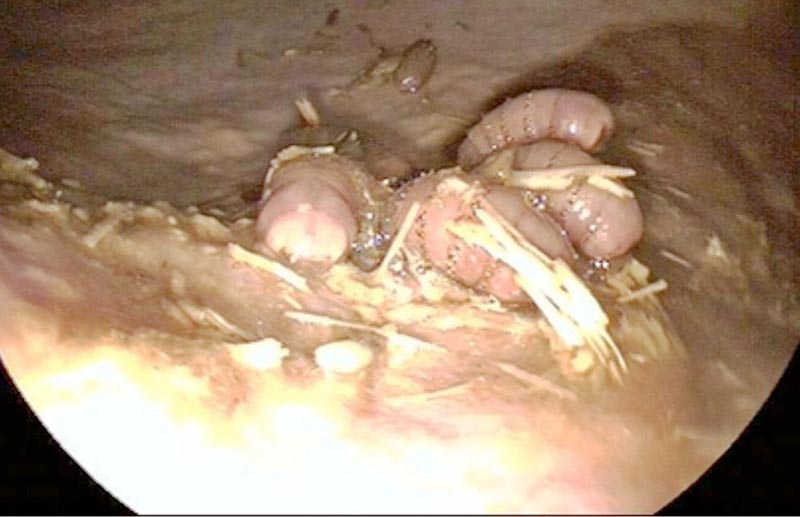
Gasterophilus species larvae (bots) are often seen attached to the stomach wall commonly adjacent to the margo plicatus (Figure 4). No evidence exists that the larvae cause discomfort and their presence has little significance other than an indicator of the horse’s anthelmintic treatment.
Ivermectin and moxidectin are generally effective against the larvae, but treatment for presence of bots alone is not recommended in light of their benign nature and increasing concerns surrounding anthelmintic resistance.