21 Nov 2016
David Rendle, Richard Newton and Debra Elton discuss equine influenza, including strain divergence, past outbreaks, its effects and the importance of surveillance and up-to-date vaccinations.

Influenza poses a constant threat and surveillance is critical to ensure strain divergence is identified and vaccines are updated accordingly.

Why worry about equine influenza (EI)? The EI virus is endemic in both the US and Europe, and results in frequent outbreaks of disease worldwide.
The 2007 outbreak in Australia demonstrated the potential for disease spread in non-vaccinated populations of horses, with more than 4,500 premises affected in an outbreak that took five months to control, even with immense government resources being deployed, at a total cost of around Aus$1 billion (£0.62 billion).
In 2003, after their jockey club gave in to pressure to drop mandatory vaccination, the racing industry in South Africa ground to a halt for three months; thousands of horses were infected – some died.
In the UK it is easy to feel complacent the equine population is sufficiently well vaccinated to protect against an outbreak; however, estimates suggest only around 40% of horses are up to date with vaccination, and this figure would need to almost double for there to be confidence in preventing an epidemic.
EI is highly infectious, with a typical incubation period of one to three days. Spread during an outbreak may, therefore, be very fast, with transmission frequently occurring before clinical signs are identified.
The first clinical sign in unvaccinated horses is marked pyrexia with rectal temperatures reaching up to 106°F. Depression and anorexia generally accompany the pyrexia, followed by combinations of serious nasal discharge, which becomes mucopurulent, lymphadenopathy and a persistent, dry, harsh, hacking cough. Myositis can also develop, resulting in muscle soreness.
In vaccinated horses, clinical disease is usually mild and can be difficult to identify. A cough and serous nasal discharge are the most likely clinical signs.
It should be remembered, it is the very rapid spread of signs, whether marked in unvaccinated or milder in vaccinated horses, within and between groups of animals, that is so distinctive about EI.
Influenza viruses contain two major surface proteins; neuraminidase (NA) and haemagglutinin (HA). Both are identified by a number. Influenza viruses that have infected horses possess either H7N7 or H3N8. The H7N7 virus has not been isolated from horses since the 1980s, is not circulating and is probably extinct in its equine forms.
H3N8 is, therefore, the only influenza virus affecting horses – at the moment.
H3N8 viruses diverged in the late 1980s into what became known as “American” and “Eurasian” strains (even though both strains were found in all three continents). Later, further divergence of American strains developed into three groups known as “Argentina”, “Kentucky” and “Florida” sublineages. Further divergence within the Florida sublineage has given rise to two clades; imaginatively termed “1” and “2”. With the exception of a few, clade 1 outbreaks at the end of 2009, all UK outbreaks, from which virus has been isolated since 2003, have been caused by Florida clade 2 viruses. Through 2015 and 2016, these have all been related viruses with a substitution at amino acid 144 of the HA protein, which was first identified in 2011.
In 2015, there were 22 confirmed outbreaks of EI across the country, from Dorset to Lanarkshire. Most affected horses had either not been vaccinated or were not vaccinated at the recommended frequency. This was a slight reduction from the 31 outbreaks recorded in 2014, with all but two of these occurring between August and December.
Fewer outbreaks have occurred in 2016. In June an unvaccinated cob gelding and unvaccinated Irish sports horse gelding developed coughing, serous nasal discharge and pyrexia approximately one week after being imported from abroad. Four vaccinated horses, with which they had direct contact, did not develop clinical signs. One week later, a three-year-old unvaccinated Irish sports horse gelding in Kent was identified as infected after it too had developed pyrexia, coughing and nasal discharge a week after being imported. Two vaccinated and one unvaccinated horse, with which it had contact, remained free from clinical signs.
In September a three-year-old unvaccinated filly, which presented with mucopurulent nasal discharge and a mild cough six days earlier, was also confirmed to be infected.
At the start of October, a positive case was also identified in Stirlingshire, and an outbreak is still being investigated.
Though outbreaks have been fewer in 2016, those that have occurred have demonstrated how susceptible we remain to sporadic outbreaks of disease, both from imported horses and the background of virus that must be circulating in the UK for spontaneous clinical cases (that cannot be traced to previous outbreaks) to develop.
The identified cases are likely to be the tip of the proverbial iceberg, as those of us who are in equine practice will be well aware, a significant proportion of horses showing signs of infectious respiratory disease are not subject to testing for influenza.
Historical precedent suggests, on average, high risk of a major outbreak about 10 years after the last. With current vaccine strains being a minimum of 9 years old and less than 50% of the equine population being vaccinated, an epidemic is a constant threat.
In 1979, a major outbreak occurred in the UK, in which vaccinated horses were affected. The outbreak caused huge disruption to the racing industry and prompted the implementation of mandatory vaccination by the jockey clubs of the UK, France and Ireland, as well as updates of vaccine strains.
Ten years later, a further epidemic occurred with large numbers of vaccinated horses being affected.
In 1993, American lineages were identified in the UK for the first time, prompting vaccines to be updated with both American and Eurasian strains.
Ten years later, in 2003, the emergence of a Florida lineage in the UK caused considerable disruption in Newmarket, where large numbers of horses, including those that were vaccinated, developed clinical disease.
In 2015, vaccination breakdowns associated with infection with clade 2 virus were recorded in Europe. Only one of the vaccines licensed in the UK contains a representative of clade 2 virus.
In March 2016, the World Organisation for Animal Health (OIE) reiterated the position it has taken since 2010 – vaccines should contain representatives of both clade 1 and 2 Florida sublineages.
The AHT, the UK OIE reference laboratory for EI virus, operates a surveillance scheme, sponsored by the Horserace Betting Levy Board (HBLB). It is free to join and offers free sampling equipment and updates on influenza outbreaks. Samples are also processed free of charge, provided a complete set of samples is received1.
Horses should be sampled if they are:
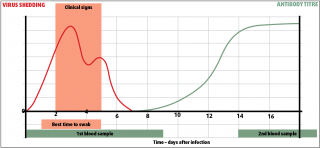
Nasopharyngeal swabs collected within one to three days of onset of clinical signs from affected horses and from close contacts that may not yet be affected, and paired blood samples collected at onset and not less than two weeks later, may be used for diagnosis (Figure 1), but swabs are required for virus isolation and genetic determination.
Samples from in-contact horses often provide valuable information as these horses are more likely to be shedding virus at the time of veterinary examination of the sentinel case, which may have ceased shedding virus. While it may be tempting to “wait and see” before collecting samples, this may miss the window during which the virus is being shed and prevent virus isolation and typing.
Keep nasopharyngeal swabs and appropriate transit media in the car and sample at the first visit. For further advice, refer to the AHT’s website2. Be aware, samples will only be processed free of charge on the HBLB scheme once the second sample has been submitted.
The virus is transmitted from infected horses by aerosol, potentially over very long distances, or in infected respiratory droplets, either direct horse-to-horse or indirectly via fomites.
If influenza is suspected, rapid diagnosis will be important in determining measures to be taken – samples should be taken immediately.
Nasopharyngeal swabs enable virus detection by quantitative PCR, which can return rapid results (same day if the lab knows the sample is arriving in the morning), and, if positive, will be put forward for genetic sequencing of the virus (clade type) and virus isolation in eggs.
Haemagglutination inhibition or single radial haemolysis performed on blood samples require paired samples at least 14 days apart, but can demonstrate rising titres and are useful in screening for the extent of outbreaks.
The virus is likely to have spread before veterinary attention is sought, so isolate suspect cases and all horses that have had contact with them in the three days preceding the first clinical signs.